
Ultrasound-guided peripheral nerve blockade in high-intensity focused ultrasound ablation for extra-abdominal desmoid tumors: a case series
Introduction
Desmoid tumors are locally invasive soft-tissue neoplasms arising from connective tissues (1). Histologically, these tumors are monoclonal fibroblastic proliferations with no potential for metastasis. Desmoid tumors pose a low risk of death but can cause complications including severe pain, swelling, deformity, loss of range of motion, bowel obstruction, or perforation. To date, there are no evidence-based or generally accepted management guidelines of unresectable desmoids. According to the latest European consensus (2), personalized management based on age, anatomic location, biological behaviors, and symptoms is recommended, including monitoring, surgery, medical therapy, radiotherapy, and local treatment. Durable local control of tumors with little function compromise and associated morbidity is the goal of management (3,4).
Local ablative treatment, being less invasive, less expensive, and requiring less anesthesia time, has been considered an alternative approach for desmoid tumors (5,6). High-intensity focused ultrasound (HIFU) is a noninvasive modality used for the treatment of localized tumors (7-9). In the past decade, some studies have reported the use of magnetic resonance imaging (MRI)-guided or ultrasound (US)-guided HIFU for the treatment of desmoid tumors (10-15). These studies have suggested that HIFU can be used safely and effectively for treating desmoid tumors to eliminate viable tumors or to provide long-lasting control of tumor growth through repeated treatments. The treatment protocol in these studies required general anesthesia or conscious sedation with the intravenous administration of fentanyl and midazolam and caused more severe adverse events, including second-degree skin burns, nerve injury, and off-target heating compared with daily repeated HIFU treatment without sedatives or intravenous analgesics (16).
US-guided peripheral nerve blockade (USg-PNB) has been widely accepted for providing local anesthesia in surgery and pain management in recent years. Many studies (17,18) have reported that USg-PNB is a safe alternative that uses minimal amounts of local anesthetic and does not require hemodynamic monitoring or extended postprocedural observation. However, no reports of using USg-PNB during HIFU treatment have been published thus far. In this paper, we describe the usefulness and effectiveness of USg-PNB in the HIFU treatment of four patients with extra-abdominal desmoid tumors.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the Institutional Ethics Committee of Shanghai Sixth People’s Hospital and with the Helsinki Declaration (as revised in 2013). Treatment options were discussed with the patients, and written informed consent was obtained from the patients or their legal guardians for publication of the case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
During the period from September 2019 to February 2023, four patients who had symptomatic desmoid tumors that were confirmed histologically (ranging from 6.7 to 19 cm in size) were referred for USg-HIFU treatment (Table 1). In order to alleviate any pain caused during the treatment, USg-PNB was administered before the HIFU treatment.
Table 1
| Case number | Age (years) | Sex | Location of tumor | Prior treatment | Maximum diameter (cm) |
|---|---|---|---|---|---|
| 1 | 38 | Male | Right forearm | Surgery, imatinib mesylate | 17 |
| 2a | 25 | Female | Left rectus abdominis | None | 6.7 |
| 2b | 25 | Female | Iliopsoas muscle | None | 7.5 |
| 2c | 25 | Female | Obturator muscle | None | 9.6 |
| 3 | 35 | Female | Left anterolateral thigh | Apatinib | 12 |
| 4 | 15 | Female | Left calf | Surgery twice, celecoxib | 19 |
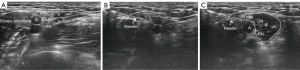
Patients were positioned in the prone or supine position to provide optimal visualization and access to the nerve. Interfascial plane blocks, a subgroup of Usg-PNB, require a different technique from that of direct nerve block, as no specific nerve is visible on US. The analgesics were injected in specific fascial planes and analgesia achieved through absorption of the injectate by the targeted nerves, with a transducer cover being employed. The skin was cleaned in compliance with the standard sterilization technique. A small-gauge standard block needle was inserted in-plane adjacent to the target nerve and was guided with real-time US visualization or into the plane between two fasciae. Beam steering was an option to enhance needle visualization. To prevent accidental vascular injection, aspiration was performed as necessary. When the needle tip was located around the epineurium or between two fasciae, the analgesics were injected under US guidance. The anesthetic was gradually injected in 1- to 2-mL portions and was visualized as hypoechoic fluid hydrodissecting and accumulating within the tissue surrounding the nerve (Figure 1) or separating two fasciae. We used a mixture of 0.75% ropivacaine (5 mL) and 1% lidocaine (5 mL) in a 1:1 ratio, which usually provides 4 to 6 hours of anesthesia sufficient for HIFU treatment procedures. The average time of the nerve block procedure was 13 minutes (range, 10 to 18 minutes). USg-PNB was performed by a radiologist with 3 years of experience in the field of musculoskeletal diagnostic and interventional US who had completed US-guided nerve block continuing educational training.
HIFU treatment was provided using the HIFUNIT-9000 (Shanghai Aishen Technology, Shanghai,China). The major parameters of therapeutic equipment were as follows: transducer diameter, 20 cm; focal length, 15 cm; frequency, 0.8 MHz; and power, 0–400 W. The echogenic change of the tumor during therapy was monitored via real-time US. Generally, the largest cross-section of the tumor is selected as the initial layer of treatment, and then treatment expands toward both ends. Each therapeutic point is subjected to 6 pulses, with a spacing of 3 mm between adjacent therapeutic points. The duration of each pulse is 200 ms, and there is a 600-ms interval between each pulse. Additionally, there is a spacing of 3 mm between adjacent treatment layers. Within each layer, sonication usually starts from deep to shallow. We set the output power of the HIFU according to the depth of the tumor. Although the treatment was performed automatically once the parameters were configured, adjustment was necessary with regard to the reaction of patients and the response to the treatment. The target region usually includes the whole tumor and a safety margin of 1 cm around the tumor with a margin of at least 1 cm from the adjacent or enveloped nerve if existed deliberately missed. Contrast-enhanced US and contrast-enhanced MRI were used to assess the extent of HIFU ablation. These procedures were performed by physicians with 2–5 years of experience in HIFU interventions. During HIFU treatment, the heart rate, blood pressure, and oxygen level of the patient were monitored. The numeric rating scale (NRS) was used to assess pain during treatment.
Case 1, a 38-year-old male, attended our HIFU clinic to undergo evaluation of a recurrent desmoid tumor in his right forearm. He had been subjected to surgical resection of the initial lesion in his forearm. The recurrence was found by MRI 6 months after resection, and he was subsequently treated with imatinib mesylate (STI-571 Glivec, Gleevec), a specific tyrosine kinase inhibitor which regulates mesenchymal cell growth and angiogenesis (2) for the following 6 months. However, the tumor continued to grow. The recurrent tumor was located in the mid-to-distal right forearm between the radius and ulna and closely adhering to the ulnar nerve and median nerve, with local bone erosion and deformity. US showed focal enlargement, hyperintensity, and altered fascicular patterns of the ulnar nerve and median nerve which were signs of neuropathy. The tumor had an irregular shape, with a maximum length of 17 cm at the time of HIFU treatment (Figure 2A). The patient complained of swelling, numbness, and pain in his right hand and forearm, with decreased grip strength and muscle atrophy. He received three HIFU treatments. US-guided brachial plexus nerve block was performed, with an axillary approach being selected for operative pain relief before HIFU treatment. All treatments were carried out smoothly and successfully. The pain was well controlled with a regional nerve block, and only a first-degree skin burn was observed on some parts of the forearm. The patient’s complaints of pain in his right hand and forearm were significantly relieved 2 days after the final treatment. MRI showed that more than 90% of the tumor had been ablated immediately after the final treatment (Figure 2B). During 29 months of follow-up, the maximum length of the tumor shrank to 3 cm (Figure 2C).

Case 2, a 25-year-old female, attended our HIFU clinic for evaluation of a desmoid tumor in the rectus abdominis, iliopsoas, obturator, and gluteus muscle around the left hip (Figure 3A). The largest lesion was in her rectus abdominis muscle as histologically confirmed by biopsy. She complained of discomfort during hip adduction and external rotation. Due to the particular location of the tumor, a resection would result in a critical functional sacrifice that was not suggested by a multidisciplinary team (MDT) specializing in the care of soft-tissue tumors. The patient refused chemotherapy and radiation therapy due to personal reasons. She received two different approaches simultaneously as suggested by the MDT: (I) HIFU treatment of lesions in the rectus abdominis and gluteus muscles and (II) watchful waiting management of smaller lesions in the iliopsoas and obturator muscles. She received two HIFU treatments separately on both lesions. Unilateral subcostal transversus abdominis plane nerve block was performed under US guidance before HIFU treatment of the lesion in the rectus abdominis. No anesthetic approach was applied to the patient during the HIFU treatment of the lesion in the gluteus muscle. The pain was controlled well in the treatment of the lesion in the rectus abdominis, and no skin burn was observed. A complaint of mild pain was encountered during the treatment of a lesion in the gluteus muscle. MRI showed that more than 80% of both tumors had been ablated (Figure 3B). During 48 months of follow-up, the volume of tumor treated with HIFU shrank by 95% (Figure 3C). On the other hand, the lesion in the iliopsoas muscle had increased from 1.5 to 9.6 cm in diameter at 33 months of follow-up. The patient then received two HIFU treatments on the lesion in the iliopsoas muscle. Transversus fascial plane nerve block and femoral nerve block were performed under US guidance before HIFU treatment of the lesion in the iliopsoas muscle. The pain was well controlled, and no skins burn were observed. MRI showed more than 60% ablation of the tumor, and during the 15 months of follow-up, the tumor in the iliopsoas muscle shrank to 6 cm in diameter with no obvious progression.
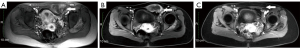
Case 3, a 35-year-old female, was diagnosed with a desmoid tumor in the deep subcutaneous fascia of the left anterolateral thigh. The initial irregular-shaped lesion was 8 cm in diameter. She went on watchful waiting management for 24 months with the lesion progressing. Subsequently, she was treated with apatinib for 2 months but discontinued treatment due to drug side effects. The tumor continued growing in the following 10 months. When she was referred to our clinic, the tumor had increased to 12 cm in diameter (Figure 4A). She complained of paresthesia of the left anterolateral thigh and experienced pain when sitting. US showed a thickened femoral lateral cutaneous nerve running through the lesion. She received three HIFU treatments. A left lateral femoral cutaneous nerve block was performed under US guidance for operative pain relief before HIFU treatment. The pain was controlled well with a regional nerve block, and only a first-degree skin burn was observed on part of the left anterolateral thigh. MRI showed more than 80% ablation of the tumor immediately after the final treatment (Figure 4B). During 1 month of follow-up, the tumor in the deep subcutaneous fascia shrank to 11 cm in diameter with no obvious progression.

Case 4, a 15-year-old female, was diagnosed with a desmoid tumor in her left calf. She had undergone surgical resection of the initial lesion. The first recurrence was detected 6 months after resection, and the recurrent tumor was surgically removed again. However, 6 months after the second resection, another recurrence was confirmed by MRI. The recurrent tumor was located at the original surgical region within the gastrocnemius and the soleus muscle with the tibial nerve being encapsulated. When she was referred to our clinic, the maximum length of the tumor had grown to 19 cm (Figure 5A). She complained of left calf pain and restricted dorsiflexion of the left foot. She received five HIFU treatments. A left popliteal tibial nerve block was performed under US guidance for operative pain relief before HIFU treatment. The pain was well controlled, and no skin burns were observed. The patient’s complaints of pain were significantly relieved by the end of the treatments. MRI performed after the treatments showed that approximately 80% of the tumor had been ablated (Figure 5B). During 2 months of follow-up, the lesion within the gastrocnemius and the soleus muscle shrank to 18 cm in diameter with no obvious progression (Figure 5C).

Discussion
Desmoid tumors are tough, ill-defined, and characterized by abundant collagen and fibroblastic cells that infiltrate the surrounding fascia and muscle. The principle of HIFU therapy is to focus the ultrasonic waves generated by the ultrasonic transducer outside the body on the lesion in the body so that the sound intensity in the focal area is sufficiently high to cause tissue coagulation necrosis. Thus far, HIFU therapy has been mostly applied to the treatment of uterine fibroids, prostate cancer, and bone metastases. A few studies (10,11,13,14) have reported HIFU-treated desmoid tumors with relatively high ablation rates, ranging from 49% to 90%. The effectiveness may be related to the histopathologic tissue type of desmoid which is similar to hyalinized uterine fibroids containing a higher proportion of collagen with a high sound absorption coefficient and sensitivity to heat. Compared with intra-abdominal desmoids, extra-abdominal tumors have an easier placement and alignment during HIFU treatment due to a lack of interference and obstruction of intestinal gas and ribs. Fit-to-shape HIFU ablation of desmoid tumors can be used to maximally preserve function. Furthermore, without concern for cumulative dose effects, multiple HIFU treatments are suitable for long-term disease control.
However, the pain during the ablation can be an obstacle to HIFU ablation. In related studies, HIFU treatments of desmoid tumors were consistently performed under general anesthesia or conscious sedation. Complications of general anesthesia and conscious sedation may include postoperative pain, nausea, vomiting, headache, feelings of sleepiness, and respiratory problems. Moreover, general anesthesia requires at least 8 hours of fasting and closer monitoring of vital signs, which is not required with the nerve block technique. In contrast, USg-PNB is associated with fewer complications and effective pain control. US examination before HIFU treatment not only acts as a HIFU pretreatment localization technique but also permits a detailed and person-specific examination of the peripheral nerve that innervates the tumor region. Moreover, US facilitates visualization of the block needle, target nerves, and local anesthetic injection which allows fast and accurate needle placement with small volumes of local anesthetic (19).
This is the first report of USg-PNB being used during HIFU treatment. We report four patients with extra-abdominal desmoid tumors treated with US-guided HIFU ablation under US-guided peripheral nerve block. The mean ablation rate of the tumor was 78% (range, 60–90%). The successful rate of single-injection nerve block was 100%. Of note, due to the relatively short treatment time and to reduce the neurotoxic of local anesthetic agents, low doses and low concentrations of anesthetic agents were applied. Patients were awake during the treatment and could be treated from different angles by performing simple position changes to improve the effectiveness of treatment. During treatment, the NRS was used to assess pain severity (Table 2). The mean NRS score was less than 1 during HIFU treatment. Patients with extra-abdominal desmoid tumors are generally at greater risk of skin burns due to the relatively short skin-to-lesion distance. In this case series, 2 out of 4 patients experienced first-degree skin burns that healed within 2 weeks. Although some studies (20,21) have reported long-term complications including sensory and/or motor deficit of the nerve caused by USg-PNB, no long-term complications of USg-PNB were observed in our cases. No patient experienced functional impairment or infection after the HIFU ablation, and all patients returned home 30 minutes after the HIFU ablation.
Table 2
| Case number | Blocked nerve | HIFU sessions (n) |
Pain during HIFU (NRS†) | Total follow-up (months) | Maximum diameter before HIFU (cm) | Maximum diameter at the end of follow-up (cm) |
Ablation ratio‡ (%) | Side effects |
|---|---|---|---|---|---|---|---|---|
| 1 | Axillary brachial plexus | 3 | 0 | 29 | 17 | 3 | >90 | First-degree skin burn |
| 2a | Transverse abdominus plane | 2 | 0 | 48 | 6.7 | 1 | 80 | None |
| 2b | Femoral nerve, transverse fascial plane | 2 | 1 | 15 | 9.6 | 6 | 60 | None |
| 3 | Lateral femoral cutaneous nerve | 3 | 1 | 1 | 12 | 11 | >80 | First-degree skin burn |
| 4 | Popliteal tibial nerve | 5 | 1 | 2 | 19 | 18 | >80 | None |
†, the pain NRS with which patients rated their pain intensity at the time according to the following scheme: no pain =0, mild pain =1–3, moderate pain =4–6, and severe pain =7–10; ‡, ablation ratio = nonenhanced tumor volume/total tumor volume on MRI after treatment. HIFU, high-intensity focused ultrasound; NRS, numeric rating scale; MRI, magnetic resonance imaging.
In our experience, USg-PNB for HIFU treatment of extra-abdominal desmoid tumors is safe and effective. Compared with general anesthesia or conscious sedation, USg-PNB not only takes less time to perform, but also provides quicker recovery, requires less medication when patients return home, and reduces the cost of treatment. Despite the limited sample size, our experience provides evidence that USg-PNB could be a useful method of anesthesia in HIFU treatment for extra-abdominal desmoid tumors and is worthy of continued investigation. Further experience with USg-PNB in HIFU treatment may confirm its suitability as an anesthetic option in HIFU treatment of other extremity soft-tissue tumors such as recurrent sarcomas.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-516/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Institutional Ethics Committee of Shanghai Sixth People’s Hospital and with the Helsinki Declaration (as revised in 2013). Treatment options were discussed with the patients, and written informed consent was obtained from the patients or their legal guardians for publication of the case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol 2012;23:562-9. [Crossref] [PubMed]
- Kasper B, Baumgarten C, Bonvalot S, Haas R, Haller F, Hohenberger P, Moreau G, van der Graaf WT, Gronchi ADesmoid Working Group. Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients' and professionals' expertise - a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer 2015;51:127-36. [Crossref] [PubMed]
- Walczak BE, Rose PS. Desmoid: the role of local therapy in an era of systemic options. Curr Treat Options Oncol 2013;14:465-73. [Crossref] [PubMed]
- von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU 3rd, DeLaney TF, et al. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012;10:951-60. [Crossref] [PubMed]
- Testa S, Bui NQ, Charville GW, Avedian RS, Steffner R, Ghanouni P, Mohler DG, Ganjoo KN. Management of Patients with Newly Diagnosed Desmoid Tumors in a First-Line Setting. Cancers (Basel) 2022.
- Goldberg D, Woodhead G, Hannallah J, Young S. Role of the Interventional Radiologist in the Treatment of Desmoid Tumors. Life (Basel) 2023.
- Yeo SY, Elevelt A, Donato K, van Rietbergen B, Ter Hoeve ND, van Diest PJ, Grüll H. Bone metastasis treatment using magnetic resonance-guided high intensity focused ultrasound. Bone 2015;81:513-23. [Crossref] [PubMed]
- Zhao WP, Chen JY, Zhang L, Li Q, Qin J, Peng S, Li KQ, Wang ZB, Chen WZ. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol 2013;82:e43-9. [Crossref] [PubMed]
- Cordeiro ER, Cathelineau X, Thüroff S, Marberger M, Crouzet S, de la Rosette JJ. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int 2012;110:1228-42. [Crossref] [PubMed]
- Ghanouni P, Dobrotwir A, Bazzocchi A, Bucknor M, Bitton R, Rosenberg J, Telischak K, Busacca M, Ferrari S, Albisinni U, Walters S, Gold G, Ganjoo K, Napoli A, Pauly KB, Avedian R. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. Eur Radiol 2017;27:732-40. [Crossref] [PubMed]
- Najafi A, Fuchs B, Binkert CA. Mid-term results of MR-guided high-intensity focused ultrasound treatment for relapsing superficial desmoids. Int J Hyperthermia 2019;36:538-42.
- Mo S, Chen J, Zhang R, Yang C, Wang T, Chen L, Chen W. High-Intensity Focused Ultrasound Ablation for Postoperative Recurrent Desmoid Tumors: Preliminary Results. Ultrasound Med Biol 2022;48:638-45. [Crossref] [PubMed]
- Zhong X, Hu X, Zhao P, Wang Y, Fang XF, Shen J, Shen H, Yuan Y. The efficacy of low-power cumulative high-intensity focused ultrasound treatment for recurrent desmoid tumor. Cancer Med 2022;11:2079-84. [Crossref] [PubMed]
- Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia 2011;27:648-53. [Crossref] [PubMed]
- Zhao WP, Han ZY, Zhang J, Yu XL, Cheng ZG, Zhou X, Liang P. Early experience: high-intensity focused ultrasound treatment for intra-abdominal aggressive fibromatosis of failure in surgery. Br J Radiol 2016;89:20151026. [Crossref] [PubMed]
- Cho JY, Kim SH, Kim SY, Moon SK, Li J. Efficacy and safety of daily repeated sonographically guided high-intensity focused ultrasound treatment of uterine fibroids: preliminary study. J Ultrasound Med 2013;32:397-406. [Crossref] [PubMed]
- Shteynberg A, Riina LH, Glickman LT, Meringolo JN, Simpson RL. Ultrasound guided lateral femoral cutaneous nerve (LFCN) block: safe and simple anesthesia for harvesting skin grafts. Burns 2013;39:146-9. [Crossref] [PubMed]
- Kim YM, Joo YB, Kang C, Song JH. Can ultrasound-guided nerve block be a useful method of anesthesia for arthroscopic knee surgery? Knee Surg Sports Traumatol Arthrosc 2015;23:2090-6. [Crossref] [PubMed]
- Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth 2005;94:7-17. [Crossref] [PubMed]
- Barrington MJ, Watts SA, Gledhill SR, Thomas RD, Said SA, Snyder GL, Tay VS, Jamrozik K. Preliminary results of the Australasian Regional Anaesthesia Collaboration: a prospective audit of more than 7000 peripheral nerve and plexus blocks for neurologic and other complications. Reg Anesth Pain Med 2009;34:534-41. [Crossref] [PubMed]
- Lauf JA, Huggins P, Long J, Al-Issa M, Byrne B, Large BP, Whitehead B, Cheney NA, Law TD Sr. Regional Nerve Block Complication Analysis Following Peripheral Nerve Block During Foot and Ankle Surgical Procedures. Cureus 2020;12:e9434. [Crossref] [PubMed]


