
Rosai-Dorfman disease of the kidney: a case description and literature analysis
Introduction
Rosai-Dorfman disease (RDD) was originally reported by Destombes in 1965 (1) and described in detail by Rosai and Dorfman in 1969 (2). RDD, also known as sinus histiocytosis with massive lymphadenopathy (SHML), is a rare non-Langerhans cell histiocytosis with an incidence of approximately 1 in 200,000 (3). Histiocytic lesions can be classified as dendritic cell disease, macrophage-related disease, or malignant histiocytic disease; macrophage-related diseases include SHML and hemophagocytic lymphohistiocytosis (HLH) (2).
A previous study has shown that RDD is a benign and self-limiting disease (4). Recently, the presence of mutations in the mitogen-activated protein kinase (MAPK) gene in patients with RDD has demonstrated that the disease is neoplastic (5). The exact etiology and pathogenesis of the disease are unclear, but the disease is associated with viral infections such as human herpes virus (HHV), Epstein-Barr virus (EBV) (6). In addition, those infected with human immunodeficiency virus (HIV) are often co-infected with RDD (7). The clinical manifestations of RDD are variable and the imaging characteristics are nonspecific. In the kidney, RDD imaging characteristics are similar to malignant renal tumors, resulting in a high rate of misdiagnosis. To the best of our knowledge, this is the first report on the characteristics of contrast-enhanced ultrasound (CEUS) of RDD compared with contrast-enhanced magnetic resonance imaging (CEMR). This case report is intended to improve the understanding of clinicians and imaging physicians by reporting on a rare RDD disease of the kidney that was misdiagnosed as renal cell carcinoma (RCC) and describing the imaging and histologic features of the disease.
Case presentation
All procedures performed in this study followed the ethical standards of the institutional and national research committees and the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review from the editorial office of this journal. The patient, a 57-year-old male, was hospitalized with ultrasound findings of renal lesions on the right kidney during a routine physical examination. He had no hematuria, dysuria, or flank pain, and had suffered from hypertension, diabetes, and gout for several years. A two-dimensional ultrasound was performed using an SC6-1 probe (Mindray Resona 7), revealing a hypoechoic lesion in the right renal sinus measuring approximately 1.9 cm × 1.3 cm. To further define the nature of the mass, a CEUS was performed. A suspension of Sonovue (Bracco, Milan, Italy) was injected through a peripheral ante cubital vein with a bolus of 0.02 mL/kg. A low mechanical index (MI =0.072) was used. Continuous dynamic observation lasted at least 3 min following bolus injection. The tumor was characterized by earlier wash-in compared to renal parenchyma, centripetal enhancement, and homogeneous hypoenhancement with a pseudocapsule sign. Non-clear cell RCC (non-ccRCC) was diagnosed based on these features (Figure 1).
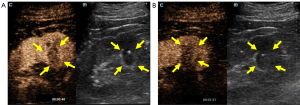
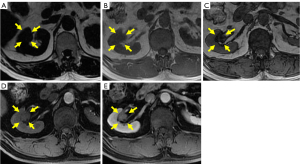
CEMR showed isoenhancement on the T1-weighted image and iso- or hypoenhancement on the T2-weighted image in the sinus region of the right kidney. The significantly decreased intensity of the lesion on the out-of-phase image indicated the presence of lipids in the lesion. A pseudocapsule was suggested by the hypoenhancement on the T2-weighted image around the mass. Mild enhancement in the lesion was observed in the cortical phase and persistent moderate homogeneous enhancement occurred in the medullary and excretory phases. These CEMR characteristics also suggested a lesion with a deficient blood supply that could be considered non-ccRCC (Figure 2A-2E).
A robot-assisted laparoscopic right partial nephrectomy was performed and pathology indicated a diagnosis of RDD. The vital signs of the patient were stable and the incisions healed well without obvious redness, swelling, or exudation. During follow-up, no abnormal cervical and inguinal lymph nodes were found in this patient, and no recurrence occurred. The most striking pathological feature of the histiocytes was emperipolesis and immunohistochemical findings were positive for S-100 and CD68 and negative for CD1a (Figure 3A-3D).

Discussion
According to the 2016 histiocytoses classification criteria, RDD, including classical, extranodal, RDD associated with neoplasia or immune disease, and unclassified RDD types, belongs to the R group of histiocytoses (8). RDD occurs mainly in the lymph nodes, with about 40% in extranodal organs (9). RDD has a predominantly single systemic involvement, in which skin, bone, and soft tissue are most frequently involved. Kidney involvement is less common, accounting for 4% of cases; the prognosis of RDD of the kidney varies among researchers (10). Similar to a study by Lai et al. (11), our follow-up results indicated renal involvement representing a benign lesion. Only 19% of RDD patients have multi-organ involvement. While RDD is mostly benign and self-limiting, the various clinical manifestations and non-specific imaging results make the diagnosis challenging and difficult to differentiate from RCC, lymphoma, and metastasis, which can cause hematuria, abdominal fullness, flank pain, ureteral obstruction, or nephrotic syndrome caused by renal vein thrombosis (10). In this case, the patient did not have any of the above clinical symptoms but had a 6-year history of gout, consistent with a previous study suggesting that patients with RDD with renal involvement tend to have a history of immune system disorders or long-term hormone use (12).
The definitive diagnosis of RDD is currently based on pathology. The characteristic manifestation is numerous enlarged histiocytes with emperipolesis—the presence of characteristic histiocytes, plasma cells, and lymphocytes, with occasional eosinophils and neutrophils, in the expanded sinuses of the enlarged histiocyte, often arranged along the perimeter of the cytoplasm (13). Immunohistochemical features are positive for the S-100 protein that is used for visualization of emperipolesis (14), positive for CD68, and negative for CD1a.
Previous studies (15,16) have shown that renal-associated RDD often involves the renal sinuses, the hilum, or the subcapsular space, and may be associated with disease in the renal lymphatic system, as the lymphatic vessels in the kidney are mainly located below the capsule and drain to the hilum (17). The lymph node predilection of RDD and the tendency of lymphoma to affect the hilum or to encase the kidney may also indirectly support this hypothesis. RDD may also result in distortion of the lymphatic collecting system but does not typically involve obstruction or vascular infiltration.
RDD involving the kidney is uncommon and often misdiagnosed as a renal malignancy, resulting in kidney loss (18). RCC is the most common solid lesion found in the kidney, accounting for approximately 3–5% of all adult cancers and approximately 90% of all kidney malignancies (19). The most common types of RCC are ccRCC (80–90% incidence) and non-ccRCC [papillary RCC (pRCC), 10–15% incidence; chromophobe RCC (chRCC), 4–5% incidence]. Current studies suggest that ccRCC and non-ccRCC have different contrast-enhanced image characteristics. Most non-ccRCCs show mainly heterogeneous hypoenhancement in CEMR and CEUS, and the pseudocapsule sign is observed less frequently in non-ccRCC than in ccRCC. Heterogeneous hyperenhancement is predominantly associated with hemorrhage, necrosis, and cystic changes in the tumor (20). In this report, both CEUS and CEMR showed iso- and hypoenhancement, consistent with the findings of Lu et al. (21) using contrast-enhanced CT and CEUS. RDD typically displays obvious lymphoid follicles with germinal centers, fibrosis, and sclerosis, while hemorrhage and necrosis are rare, which is reflected in image homogeneity. Heterogeneity of the tumor can indicate malignancy and degree of differentiation; homogeneity in RDD indirectly reflects the low aggressiveness of the disease (22) and hypoenhancement is due to fewer vascular components in the lesion.
The pseudocapsule sign is commonly seen in early low-grade RCC, mainly due to tumor growth causing compression, ischemia, and necrosis of the adjacent parenchyma with deposition of fibrous tissue. In this case, the appearance of a pseudocapsule sign was observed in both CEUS and CEMR, but the presence of a pseudocapsule sign has not been previously indicated in published literature. One study (23) showed a greater degree of fibrosis and RDD histiocytes on an extranodal site, suggesting extranodal RDD more often presents as fibrosis and less often as histiocytosis. The pathological findings in our case showed myofibroblast and fibrous tissue hyperplasia, with a large number of lymphocytes and plasma cells infiltrated into the renal tissue, which corroborated with previous studies and may explain the appearance of a pseudocapsule sign due to the distribution of fibrous tissue around the lesion.
There are no universal standards for the treatment of RDD with kidney involvement. The usual treatment modalities are surgery, radiotherapy, chemotherapy, and hormonal therapy (24). Combined with a previous study and follow-up results, we believe that RDD has a good prognosis and renal involvement is a benign lesion. Surgical resection may lead to overtreatment, and thus observation is recommended (11). Furthermore, the differential diagnosis of renal malignancy may be improved if further studies of imaging examinations can validate signs observed in this study that, when combined with medical history, can differentiate RDD from RCC. Such improved differential diagnosis would mean resection of the kidney could be avoided.
In summary, as shown in this case, RDD involving an isolated kidney exhibits homogeneous hypoenhancement and appears in the renal sinus, hilum, and subcapsule of the kidney. Combined with the patient’s history and laboratory tests, clinicians can make a comprehensive diagnosis and manage the patient accordingly to avoid unnecessary surgery.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-773/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Destombes P. Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali. (4 cases). Bull Soc Pathol Exot Filiales 1965;58:1169-75.
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol 1969;87:63-70.
- Mahzoni P, Zavareh MH, Bagheri M, Hani N, Moqtader B. Intracranial ROSAI-DORFMAN Disease. J Res Med Sci 2012;17:304-7.
- Ravindran A, Rech KL, How I. Diagnose Rosai-Dorfman Disease. Am J Clin Pathol 2023;160:1-10. [Crossref] [PubMed]
- Chang L, Qiao B, Cai H, Lin H, Duan MH, Li J, Zhou DB, Goyal G, Sun CY, Cao XX. Clinical phenotypes, molecular analysis, and outcomes of patients with Rosai-Dorfman disease. Leukemia 2023;37:2297-300. [Crossref] [PubMed]
- Quadrelli C, Barozzi P, Riva G, Vallerini D, Zanetti E, Potenza L, Forghieri F, Luppi M. β-HHVs and HHV-8 in Lymphoproliferative Disorders. Mediterr J Hematol Infect Dis 2011;3:e2011043. [Crossref] [PubMed]
- Laudin GE, Lakha AB, Dullabh N, Mohanlal R, Jassat R, Waja MF, Philip V. A meta-analysis of cases of Rosai Dorfman disease reported on the African continent and a description of two cases from a tertiary academic hospital in Johannesburg, South Africa. Pan Afr Med J 2023;45:130. [Crossref] [PubMed]
- Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016;127:2672-81. [Crossref] [PubMed]
- Mazzariol M, Peyronel F, Fagni F, Minervini A, Santi R, Agostini S, Vaglio A. Kidney involvement in Rosai-Dorfman disease. Kidney Int 2023;103:231-2. [Crossref] [PubMed]
- Abla O, Jacobsen E, Picarsic J, Krenova Z, Jaffe R, Emile JF, Durham BH, Braier J, Charlotte F, Donadieu J, Cohen-Aubart F, Rodriguez-Galindo C, Allen C, Whitlock JA, Weitzman S, McClain KL, Haroche J, Diamond EL. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood 2018;131:2877-90. [Crossref] [PubMed]
- Lai KL, Abdullah V, Ng KS, Fung NS, van Hasselt CA. Rosai-Dorfman disease: presentation, diagnosis, and treatment. Head Neck 2013;35:E85-8. [Crossref] [PubMed]
- Wang F, Xu J, Chen J, Fu H, Wang Z, Cai D, Kang X, Zhang BB, Matz EL, Zhang Y, Wang W. A case report of Rosai-Dorfman disease with kidney involvement. J Xray Sci Technol 2018;26:141-6. [Crossref] [PubMed]
- Liu G, Wang H, Yang Z, Tang T, Zhang S. Is it a Metastatic Disease: A Case Report and New Understanding of Rosai-Dorfman Disease? Am J Dermatopathol 2016;38:e72-6. [Crossref] [PubMed]
- Aoyama K, Terashima K, Imai Y, Katsushima N, Okuyama Y, Niikawa K, Mukada T, Takahashi K. Sinus histiocytosis with massive lymphadenopathy. A histogenic analysis of histiocytes found in the fourth Japanese case. Acta Pathol Jpn 1984;34:375-88. [Crossref] [PubMed]
- Kugler A, Middel P, Gross AJ, Kallerhoff M, Ringert RH. Unusual bilateral renal histiocytosis: extranodal variant of Rosai-Dorfman disease. J Urol 1997;157:942. [Crossref] [PubMed]
- Bain ES, Kinney TB, Gooding JM, Casola G, Ysrael MZ. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman Disease): a rare cause of bilateral renal masses. AJR Am J Roentgenol 1999;172:995-6. [Crossref] [PubMed]
- Gray H. Gray’s anatomy: the anatomical basis of medicine and surgery. 38th ed. New York: Churchill Livingstone; 1995.
- Peng N, Wang X, Zhang Z, Fu S, Fan J, Zhang Y. Diagnosis value of multi-slice spiral CT in renal trauma. J Xray Sci Technol 2016;24:649-55. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Liu H, Cao H, Chen L, Fang L, Liu Y, Zhan J, Diao X, Chen Y. The quantitative evaluation of contrast-enhanced ultrasound in the differentiation of small renal cell carcinoma subtypes and angiomyolipoma. Quant Imaging Med Surg 2022;12:106-18. [Crossref] [PubMed]
- Lu A, Xie X, Han J, Yu J, Qin X, Xu P. Asymptomatic Rosai-Dorfman-Destombes disease presenting as isolated bilateral perinephric infiltration: a case report and review of the literature. Transl Androl Urol 2023;12:347-52. [Crossref] [PubMed]
- Gulati M, King KG, Gill IS, Pham V, Grant E, Duddalwar VA. Contrast-enhanced ultrasound (CEUS) of cystic and solid renal lesions: a review. Abdom Imaging 2015;40:1982-96. [Crossref] [PubMed]
- Wright DH, Richards DB. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case with widespread nodal and extra nodal dissemination. Histopathology 1981;5:697-709. [Crossref] [PubMed]
- Magableh HM, Jaber HD, Magableh AM, Alrabiah MA, Dahhan AF, Azzam AZ, Amin T. Rosai-Dorfman Disease: Case Series and Literature Review. Cureus 2023;15:e35193. [Crossref] [PubMed]


