
Kimura disease with optic nerve and intracranial involvement: a case description
Introduction
Kimura disease (KD), also known as eosinophilic granuloma, is a rare chronic lymphoproliferative inflammatory disease. The main clinical manifestation of KD is the presence of long-standing and recurrent painless subcutaneous soft tissue masses in the head and neck region, which may be accompanied by peripheral blood eosinophilia and elevated serum immunoglobulin E (IgE) levels. However, there have been a few reported cases of KD occurring in other areas, such as the mediastinum and orbit (1,2). Due to atypical clinical symptoms and non-specific laboratory and imaging findings, it is easily missed or misdiagnosed, and pathological examination is the gold standard for clinical diagnosis. Here, we report for the first time a case of KD involving the brain, optic nerve, and lymph nodes.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 22-year-old female of Chinese Han ethnicity was admitted to our neurosurgery department with a history of gradual vision loss and accompanying periocular pain in both eyes over the course of 6 months. Additionally, she had experienced four episodes of seizures characterized by altered consciousness, limb twitching, and trismus, along with occasional headaches and dizziness, within the past 2 months.
Past medical history
In 2020, the patient experienced lower limb edema and was diagnosed with nephritis at a local hospital. The symptoms improved after treatment and did not recur thereafter. She had no history of chronic illness, mental illness, or family history of inherited diseases.
Physical examination
The patient exhibited strabismus in both eyes, bilateral visual impairment, and temporal hemianopia. The visual acuity examination yielded the following results: binocular vision was at the level of light perception. No ocular protrusion was noted. The patient’s pupils were symmetrical, round, and 3 mm in diameter, demonstrating sensitivity to light reflex. Enlarged and fused lymph nodes were palpable in the right axilla, measuring approximately 6 cm × 3 cm. The lymph nodes had a firm consistency, good mobility, and well-defined borders.
Laboratory investigations
Laboratory investigations revealed markedly elevated eosinophil count of 1.59×109/L [normal range, (0.02–0.52)×109/L], increased eosinophil percentage of 15.6% (normal range, 0.4–8%), and elevated IgE level of 507 IU/mL (normal range, <100 IU/mL). Urine examination showed proteinuria (++) and occult blood (+). The patient tested positive for antinuclear antibodies (ANAs). The erythrocyte sedimentation rate (ESR) was elevated at 28 mm/h (normal range, 0–20 mm/h). The tumor markers [alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen (CA)125, CA153, CA199, cytokeratin 19 fragment (CYFRA21-1), neuron-specific enolase (NSE), pro-gastrin-releasing peptide (pro-GRE), human epididymis protein 4 (HE4), β-human chorionic gonadotropin (β-hCG), and squamous cell carcinoma antigen (SCCA)] tested were all negative.
Ultrasonography
Ultrasonography of the axillary lymph nodes revealed a 6 cm × 3 cm cystic-solid mass in the right axilla with regular shape and clear borders.
Imaging studies
The cranial computed tomography (CT) scan revealed a slightly higher density lesion in the right frontal lobe with surrounding patchy areas of slightly lower density (Figure 1A). The cranial magnetic resonance imaging (MRI) showed multiple nodular fused lesions in the right frontal lobe, with isointense T1 and short T2 signals. The fluid-attenuated inversion recovery (FLAIR) sequence demonstrated low signal intensity, and the diffusion-weighted imaging (DWI) sequence showed low signal intensity. The lesions exhibited significant enhancement after contrast administration. Surrounding the lesions, there were patchy and uneven long T1 and T2 signals, with no enhancement observed (Figure 1B-1J). The magnetic resonance spectroscopy (MRS) image of the lesion showed a significant elevation of the choline (Cho) peak and a decrease in the N-acetyl aspartate (NAA) peak. The NAA/creatine (Cr) ratio was 0.59, the Cho/Cr ratio was 1.98, and the Cho/NAA ratio was 3.37. A lipid-lactate peak was visible at 1.33 ppm. The spectral lines suggested that the lesion was likely a malignant tumor (Figure 1K).
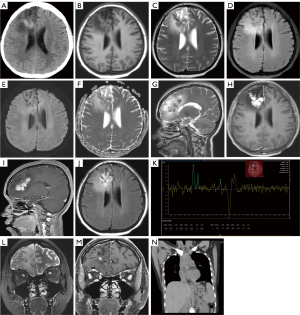
Based on the imaging findings, differential diagnosis should consider a tumor or inflammatory granuloma in the right frontal lobe. The orbital MRI showed thinning of the bilateral optic nerves and enhancement of the bilateral sheaths, which was more pronounced on the left side (Figure 1L,1M). The chest (heart, lungs, and mediastinum) CT revealed multiple enlarged lymph nodes in the right axilla, varying in size. The largest lymph node measured approximately 4.3 cm × 3.5 cm × 6.2 cm (anteroposterior × transverse × craniocaudal dimensions) (Figure 1N).
Pathological findings
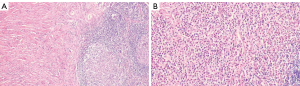
After multidisciplinary consultation, it was recommended to perform a right axillary lymph node excisional biopsy, and the pathological findings revealed local disruption of lymph node architecture. Some areas showed proliferative lymphoid follicles, whereas other areas demonstrated replacement of normal lymphoid structure with proliferative hyaline fibrous tissue. Within the lymph node, there was diffuse infiltration of abundant eosinophils accompanied by focal spindle cell proliferation (Figure 2A,2B), consistent with KD.
Diagnosis
In this case, there were multiple fused nodular lesions in the right frontal lobe, along with significant enlargement of the right axillary lymph node, and elevated levels of blood eosinophils and IgE. Taken together, the clinical history, laboratory tests, imaging findings, and histopathological examination indicated a high probability that the right frontal lobe and optic nerve involvement were related to KD.
Treatment process
Currently, the treatment options for KD primarily include surgical intervention, radiation therapy, oral corticosteroids, and immunosuppressive agents (3).
After obtaining consent from the patient and her family, the patient underwent diagnostic treatment, followed by observation of the progression of the brain lesion. The patient took 36 mg/day of methylprednisolone for a total of 3 weeks, afterward, she switched to 45 mg/day of prednisone, reducing by 5 mg every 2 weeks until reaching 10 mg to maintain control of the condition, and cyclophosphamide was added with intravenous infusion every 6 weeks.
Follow-up and outcomes
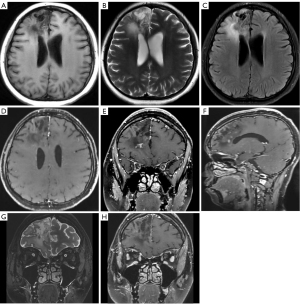
The patient was followed up for a period of 5 months, during which a cranial MRI showed a significant reduction in the size of the right frontal lobe lesion compared to before, and the degree of enhancement was significantly reduced (Figure 3A-3F). No recurrent masses were found on physical examination in the axilla or other peripheral areas. The patient’s peripheral blood eosinophil count and serum IgE returned to normal levels, but with a slight increase in eosinophils as the steroid dose was gradually reduced. Urine examination showed persistent proteinuria (2+) and hematuria (1+). The patient reported a significant improvement in vision during the first 3 months of treatment, which stabilized thereafter but did not return to normal. Upon admission, the patient had only light perception in both eyes. The most recent examination showed a visual field width of approximately 0.5 m in the left eye and approximately 3 m in the right eye. During the treatment period, there were no significant changes observed in the imaging of the bilateral optic nerves (Figure 3G,3H).
Discussion
KD, also known as eosinophilic hyperplastic lymphogranuloma, is a rare and idiopathic chronic inflammatory disorder (4). It predominantly affects Asian males, particularly young adults (20–40 years old), with a significantly higher incidence in males compared to females (5). This case involved a young female, which is even less common. The disease has a chronic course and commonly presents as painless unilateral swelling in the head and neck region. It can involve subcutaneous soft tissues, glands, and lymph nodes and is often associated with elevated levels of eosinophils and IgE. A review of the literature disclosed a high frequency of renal involvement in KD. Out of 175 reported cases of the disease, 21 (12%) were found to have proteinuria including 13 who had the nephrotic syndrome (6) Histologically, the disease is characterized by vascular proliferation, abundant eosinophil infiltration, lymphocyte proliferation with the formation of follicles, and fibrous tissue hyperplasia as the basic pathological changes.
In imaging, KD can be classified into two types based on its morphological features: nodular type with clear borders and diffuse type with unclear borders (7). Nodular lesions exhibit relatively homogeneous signals, with uniform high signal intensity on T2-weighted images (T2WIs) and significant homogeneous enhancement on contrast-enhanced scans. Conversely, diffuse lesions have heterogeneous and variable signals on T2WI, ranging from low to high signal intensity, and show varying degrees of enhancement. They may also exhibit mild to moderate delayed enhancement within low-signal fibrous structures. Studies have found that the T2WI signal and enhancement pattern of the lesions are associated with the degree of vascular proliferation and the proportion of fibrous components. Therefore, some researchers believe that the diffuse type is a continuation of the nodular type, with an increase in fibrous components leading to decreased T2WI signal intensity and less pronounced enhancement compared to the nodular type (7-9). In this case, the patient’s lesion was located in the right frontal lobe and appeared as multiple nodules with fused low signal intensity on T2WI, showing significant enhancement on contrast-enhanced scans, indicating a relatively prominent fibrous component in this particular case. Although MRS is of great value in the differentiation of brain tumors and intracerebral inflammatory lesions, there are also articles showing that encephalitis can occasionally show changes in MRS similar to those of tumors, that is, the NAA peak decreases and the Cho peak increases. However, the reduction of NAA peak and the increase of Cho peak in encephalitis are generally far less obvious than tumors. Since NAA fluctuates greatly in encephalitis patients, there are occasional cases in which the NAA of some severe viral encephalitis can even decrease more than the tumor, and encephalitis may be misdiagnosed as a tumor (10).
KD, although benign, is prone to recurrence, and there is currently no standardized treatment protocol. Clinically, surgical excision combined with hormone therapy and/or low-dose radiation therapy is often employed, and some researchers believe that combination therapy yields the best therapeutic effect with the lowest recurrence rate (11). In this case, surgical excision of the right axillary lymph node was performed, followed by adjunctive glucocorticoid therapy. The lesion in the right frontal lobe significantly reduced in size, and there was an improvement in visual acuity in both eyes. Therefore, the diagnosis of KD was considered. Additionally, to prevent disease recurrence during the gradual tapering of glucocorticoid therapy, immunosuppressive agents were added. After 5 months of treatment, the patient’s condition improved significantly but was not completely resolved.
A noteworthy limitation of this case report pertains to the absence of a pathological examination of the brain lesion. Although the absence of such an examination leaves room for uncertainty regarding the precise etiology or nature of the lesion, it is important to note that the diagnostic treatment presented in this study is still valuable in enhancing our understanding of the origin and characteristics of the brain lesion under investigation. The findings and insights garnered from this study not only provide valuable clinical information to aid in the management of similar cases but also offer potential avenues for future research.
In this case, the involvement of the right frontal lobe and optic nerve was noted as a rare manifestation of KD. This suggests that for patients with a prolonged course of intracranial or optic nerve mass lesions, accompanied by localized lymphadenopathy and elevated peripheral blood eosinophils and IgE levels, imaging findings of nodular or patchy abnormal structures with clear or unclear borders, and the presence of low signal intensity within them, showing progressive enhancement after contrast administration, the possibility of KD should be considered.
Acknowledgments
Funding: The study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-852/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu S, Deng L, Wang G, Li J, Wang J, Wen Z. Kimura's disease presenting as a posterior mediastinal mass complicated with relapsing polychondritis. Rheumatology (Oxford) 2022;61:e67-8. [Crossref] [PubMed]
- Fu KK. Orbital Kimura's disease. J R Soc Med 1993;86:234-5. [Crossref] [PubMed]
- Lee CC, Feng IJ, Chen YT, Weng SF, Chan LP, Lai CS, Lin SD, Kuo YR. Treatment algorithm for Kimura's disease: A systematic review and meta-analysis of treatment modalities and prognostic predictors. Int J Surg 2022;100:106591. [Crossref] [PubMed]
- Kakehi E, Kotani K, Otsuka Y, Fukuyasu Y, Hashimoto Y, Sakurai S, Hirotani A, Simizu K, Fujita R, Shoji K, Adachi S, Matsumura M. Response to: Kimura's disease: effects of age on clinical presentation. QJM 2020;113:383. [Crossref] [PubMed]
- Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 2004;28:505-13. [Crossref] [PubMed]
- Yamada A, Mitsuhashi K, Miyakawa Y, Kosaka K, Takehara K, Iijima M, Tanaka K, Shibata S. Membranous glomerulonephritis associated with eosinophilic lymphfolliculosis of the skin (Kimura's disease): report of a case and review of the literature. Clin Nephrol 1982;18:211-5.
- Gopinathan A, Tan TY. Kimura's disease: imaging patterns on computed tomography. Clin Radiol 2009;64:994-9. [Crossref] [PubMed]
- Park SW, Kim HJ, Sung KJ, Lee JH, Park IS. Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol 2012;33:784-8. [Crossref] [PubMed]
- Zhang R, Ban XH, Mo YX, Lv MM, Duan XH, Shen J, Li JP, Liu XW, Xie CM. Kimura's disease: the CT and MRI characteristics in fifteen cases. Eur J Radiol 2011;80:489-97. [Crossref] [PubMed]
- Peeraully T, Landolfi JC. Herpes encephalitis masquerading as tumor. ISRN Neurol 2011;2011:474672. [Crossref] [PubMed]
- Chang AR, Kim K, Kim HJ, Kim IH, Park CI, Jun YK. Outcomes of Kimura's disease after radiotherapy or nonradiotherapeutic treatment modalities. Int J Radiat Oncol Biol Phys 2006;65:1233-9. [Crossref] [PubMed]


