
Multi-slice computed tomographic analysis and identification of remarkable anatomical types of segmental bronchi in bilateral inferior lobes: based on extensive data
Introduction
Respiratory gases are transported by the tracheobronchial tree, which originates in the larynx and consists of the trachea, segmental bronchi, and bronchioles (1). For accurate interpretation of radiological images and air bronchograms, it is essential to have a precise understanding of the morphology of the bronchopulmonary segments (2).
The Boyden categorization of bronchi and the international taxonomy of bronchial anatomy, which were both published prior to the advent of computed tomography (CT) in 1950 and 1955, respectively, provide an excellent overview of the normal bronchial structure and its variants (3-5). The segmental bronchial branching patterns, however, were not addressed by these classifications. In addition to making it harder for surgeons to precisely assess the bronchial structure, such nomenclatures can make it more complicated to remove a single segmental bronchus (6).
Gray’s Anatomy (7) provides a detailed description of the tracheobronchial tree, focusing on the typical anatomical structure of the bronchi and excluding any variations that may exist. This can sometimes pose challenges for anatomists when trying to identify the bronchi in research samples. However, understanding the variations in the development of the bronchial tree is crucial (5,8). Around the fourth week of gestation, certain cells from the upper part of the foregut produce a tiny bud called the respiratory diverticulum, which marks the start of the development of the tracheobronchial system. This bud then gives rise to the right and left lung buds around the 26th to 28th day of embryonic development. Following the maturation of these lung buds into primary bronchi, which appear in a sympodial pattern at approximately 30 to 32 days of embryonic development, the five lobar bronchi appear. During 32 to 34 days of embryonic development, the lobar bronchi eventually branch out and form the segmental bronchi, during which bronchial variations may also occur (9).
The ability to accurately assess and categorize the branching patterns of bronchial segments must therefore be improved. This is crucial for improving the quality of radiological imaging, identifying bronchial branches in anatomical specimens, and making bronchoscopies and pulmonary segmentectomies easier to perform (6,10).
Up until now, various techniques, such as anatomical specimens (11), bronchography (12), transverse CT (13,14), and bronchoscopy (8), have been devised to acquire knowledge about the branching patterns of segmental bronchi. Currently, there is a lack of agreement on the most effective approach in assessing and classifying these branching patterns. Healthcare professionals need basic anatomical knowledge to better comprehend the bronchial branching patterns and their variations, as innovative surgical procedures like segmental pneumonectomy are used in clinical practice. A distinct bronchial categorization of each lung segment can give medical experts more precise instructions (15). Thus, additional anatomical investigation is essential to explore and characterize typical bronchial patterns and discover possible variations. For radiological examinations, multislice Somatom Definition Flash CT has been employed recently, which offers several image reconstruction techniques with outstanding resolution and provides a precise method for studying bronchial segments (16). The dual-source flash CT technology allows for fast scanning and reduces the impact of heart and lung activity on image density. Additionally, it can produce highly detailed images that improve diagnostic accuracy and offer a reliable way to study the anatomy of the bronchial tubes at a segmental level. It is worth noting that the Siemens syngo.via post-processing workstation can generate high-quality three-dimensional (3D) and virtual bronchoscopy (VB) images (17-21).
In recent times, the COVID-19 pandemic has had a profound impact on a global scale, leading to a substantial rise in both illness and death rates. Although widely used diagnostic methods like chest X-rays (21), CT scans, and bronchoscopies (22) are useful, there is still a demand for a reliable tool that can accurately detect and identify fibrotic changes in lung tissue. Therefore, the present investigation employing multi-slice Somatom Definition Flash CT has the potential to offer healthcare practitioners a novel option to rapidly assess the extent and pattern of the disease. As a reference, it can also be used for procedures like segmental resection, bronchoscopy, and bronchoalveolar lavage (BAL).
Regarding this, the objective of the current investigation was to investigate the morphological variations of segmental bronchi in the bilateral inferior lobes through the utilization of 3D and VB images acquired from multi-slice computed tomography (MSCT) scans. Furthermore, the study aimed to identify any potential disparities between sexes in the distribution of bronchial branches within a substantial portion of the study population. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-213/rc).
Methods
Study sample
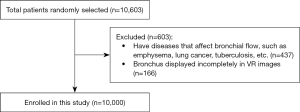
The current investigation was authorized by the Research Center of Sectional and Imaging Anatomy, Cheeloo College of Medicine, Shandong University, Jinan, China. The study was approved by the Institutional Ethics Committee of the School of Basic Medicine of Shandong University (No. ECSBMSSDU2018-1-050). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of this study. The research subjects were chosen randomly from Chinese individuals who presented with cough, dyspnea, and hemoptysis complaints between January 2020 and December 2022. The analysis conducted in our study utilized the Somatom Definition Flash CT images obtained from a sample of 10,000 individuals following the inclusion criteria as follows: (I) patients underwent routine chest CT; (II) willingness to participate in this clinical study (III) CT showed normal bronchial structures; (IV) The image quality complies with post-processing and diagnostic requirements.
The exclusion criteria were as follows: (I) patients with comorbid serious underlying diseases (such as emphysema, lung cancer, tuberculosis, etc.); (II) bronchus displayed incompletely in volume rendering (VR) images.
The study population consisted of 5,428 male participants, ranging in age from 3 to 91 years, with a mean age of 49.57 years and a standard deviation of 13.9 years. Additionally, there were 4,572 female participants, also ranging in age from 3 to 91 years, with a mean age of 49.67 years and a standard deviation of 13.3 years. The inclusion and exclusion criteria are displayed in Figure 1.
Imaging technique
The standard thoracic scanning for all individuals was conducted using the Siemens dual-source, multi-slice Somatom Definition Flash CT scanner (Forchheim, Germany) with the specified scanning settings. The parameters used in the study were as follows: collimation of 64×0.6 mm, frame rotation time of 0.33 s/r, scanning time of 4.32 s, interval of 5 s between scans, tube voltage ranging from 80 to 120 kV, reference tube current of 137 mAs, reconstruction slice thickness and interval both set at 1.0 mm, and a standardized mediastinal window. The scanning range encompassed the cranial to caudal direction, specifically spanning from the larynx to the lower edge of the diaphragm. This scanning procedure was conducted with all participants in a supine position.
Image analysis
The acquired data were transferred to the Siemens syngo.via client software to generate bronchial 3D and VB images. The scans were thoroughly evaluated and analyzed by two highly competent radiologists who possess specialized knowledge in sectional radiological anatomy. After careful examination, both radiologists arrived at a unified decision. The categorization of segmental bronchi was conducted by considering their branching direction, extension orientation, and the total number of segmental branches inside a 3D bronchial tree. Due to the rotational capability of the bronchial tree in 3D space, the viewpoint of the 3D image was frequently altered to prevent blurring of the targeted area or obstruction of the line of sight caused by neighboring bronchi.
The VB images were constructed by selecting a particular bronchus, applying a virtual endoscope to a sectional image of the CT lung window, and transforming it into a simulated bronchoscope image. Moreover, the nomenclature and numerical designation of segmental bronchi were determined by their spatial positioning and anatomical structure. The segmental bronchi were categorized based on whether they were bifurcated, trifurcated, tetrafurcated, or had two apical lobar bronchi on the 3D bronchial tree and in VB images.
Statistical analysis
The branch types of the segmental bronchus in the inferior lobes of the right and left lungs, as well as the medical record number, sex, age, and gender, were recorded. The data were analyzed with IBM SPSS Statistics 22.0 software (IBM Corporation, Armonk, NY, USA). The cross-tabulation method was initially employed to determine the component ratio of the different types of bronchial branches in the inferior lobes on both sides. The Pearson chi-square test was subsequently employed to evaluate the statistical significance of the association between sex and bronchial branch types by comparing the total composition ratio of these kinds in males and females.
Results
The thoracic CT images were thoroughly analyzed to identify the distinct forms of segmental bronchial branching seen in the inferior lobes on both sides. The task of classifying bronchial branching patterns presents difficulties when relying on manual assessment by radiologists, as this process necessitates the mental reconstruction of a 3D anatomical model based on several 2D sections. Therefore, the branching patterns of bronchi might be fully understood by a single 3D image of a bronchial tree or VB.
Bronchial tree of the right inferior lobe (RIL)
The right inferior lobar bronchus gives rise to the superior segmental bronchus (B6) independently, while extending into the main stem of basal segmental bronchi. Four main anatomical branching patterns of the right basal segmental bronchi were identified (Figures 2-6 and Table 1). The most common pattern (type 1) was observed in 7,144 cases (71.44%), which involved the basal segmental bronchi trifurcating into the medial basal segmental bronchus (B7), anterior basal segmental bronchus (B8), and a common stem of lateral and posterior segmental bronchus (B9+10) (Figure 2A,2B). Type 2, found in 1,606 cases (16.06%), showed the basal trunk bronchi trifurcating into B7, a common stem of B8+9, and B10 basal segments (Figure 3A,3B). Type 3, present in 740 cases (7.40%), displayed the basal trunk bifurcating into an inner anteromedial (B7+8) and an outer posterolateral (B9+10) stem (Figure 4A,4B). Type 4, seen in 510 cases (5.10%), involved the basal trunk bifurcating into B7 and a common stem of B8+9+10 segmental bronchi (Figure 5A,5B). The data shown in Figure 6 provide information on the distribution of these bronchus branch types in the right inferior lobar bronchus based on gender (male/female) in terms of both percentage and proportion ratios.
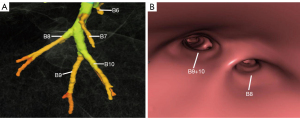
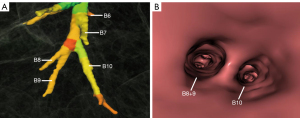
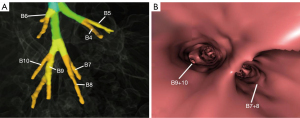


Table 1
| Classification | Pattern | Total (n=10,000) | Male (n=5,428) | Female (n=4,572) | χ2 | P value |
|---|---|---|---|---|---|---|
| Type 1 | B6, B7, B8, B9+10 | 7,144 (71.44) | 4,062 (74.83) | 3,082 (67.41) | 67.036 | <0.001* |
| Type 2 | B6, B7, B8+9, B10 | 1,606 (16.06) | 745 (13.73) | 861 (18.83) | 48.011 | <0.001* |
| Type 3 | B6, B7+8, B9+10 | 740 (7.40) | 393 (7.24) | 347 (7.59) | 0.442 | 0.506 |
| Type 4 | B6, B7, B8+9+10 | 510 (5.10) | 228 (4.20) | 282 (6.17) | 19.850 | <0.001* |
Data are presented as n (%). *, P<0.05 was considered statistically significant. The composition ratios of the bronchial branch types 1, 2, and 4 indicate a significant relationship between both sexes.
Bronchial tree of the left inferior lobe (LIL)
The superior segmental bronchus (B6) branched discretely from the posterior wall of the left inferior lobar bronchus, and then the left inferior lobar bronchus grew into the main stem of basal segmental bronchi. Four main anatomical branching patterns of the left basal segmental bronchi were found (Figures 7-11 and Table 2). The predominant type, documented in 8,289 cases (type 1, 82.89%), entails the bifurcation of the basal segmental bronchi into the common stem of medial basal segmental bronchus (B7) and anterior basal segmental bronchus (B8), as well as the common stem of lateral basal bronchus (B9) and posterior basal segmental bronchus (B10) (Figure 7A,7B). In 1,353 cases (type 2, 13.53%), the basal trunk bronchus trifurcated into a common stem of B7+8, B9, and B10 basal segments (Figure 8A,8B). In 288 cases (type 3, 2.88%), the basal segmental bronchi trifurcated into a B7, with the common stem bifurcating into B8 and B9 (B8+9) segments, and B10 (Figure 9A,9B). In 70 cases (type 4, 0.70%), the basal trunk bifurcated into B7 and a common stem of B8+9+10 segments (Figure 10A,10B). The data displayed in Figure 11 indicate the distribution of bronchus branch types in the LIL, categorized by gender (male/female), in terms of both percentage and proportion ratios.


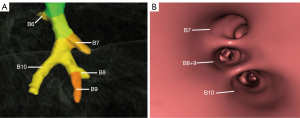
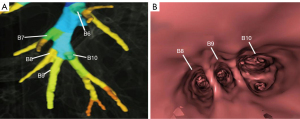

Table 2
| Classification | Pattern | Total (n=10,000) | Male (n=5,428) | Female (n=4,572) | χ2 | P value |
|---|---|---|---|---|---|---|
| Type 1 | B6, B7+8, B9+10 | 8,289 (82.89) | 4,476 (82.46) | 3,813 (83.40) | 1.538 | 0.215 |
| Type 2 | B6, B7+8, B9, B10 | 1,353 (13.53) | 754 (13.89) | 599 (13.10) | 1.322 | 0.250 |
| Type 3 | B6, B7, B8+9, B10 | 288 (2.88) | 161 (2.97) | 127 (2.78) | 0.315 | 0.575 |
| Type 4 | B6, B7, B8+9+10 | 70 (0.70) | 37 (0.68) | 33 (0.72) | 0.058 | 0.810 |
Data are presented as n (%). The composition ratios of the bronchial branch types 1–4 indicate a non-significant relationship between both sexes (P>0.05).
In addition, this study was the first to compare male and female groups to explore any potential variations in bronchial branches. The fraction of bronchial branches in the RIL showed a significant sex-related difference (P<0.05) (Table 1). Nevertheless, the analysis did not reveal any statistically significant differences between the sexes in terms of the proportion of bronchial branches in the LIL (P>0.05) (Table 2).
Discussion
The advances in interventional pulmonology require a thorough understanding of the anatomy of the bronchial system, which has expanded significantly in recent years due to the discovery of various branching types. The origins and numbers of these branches may exhibit variability among individuals, hence posing challenges for medical professionals in achieving a comprehensive understanding of the precise anatomy for diagnostic purposes and the application of optimal treatment strategies. The present work has provided novel findings regarding the existence of bronchosegmental branching patterns in a significant number of the study participants. These patterns were effectively assessed using MSCT-based 3D reconstruction and VB techniques. To fully acknowledge the various bronchial branching patterns, an ideal nomenclature is required to address this matter, which is only possible if we first establish a clear understanding of the past research methodologies and their limitations.
Several imaging techniques have been developed to investigate the course and distribution of bronchial branches, thereby facilitating accurate diagnostic and surgical planning. These techniques include dissection (11), bronchography (12,23), transverse CT (13,14), spiral CT (18,24), and conventional fiberoptic bronchoscopy (8).
Dissection is the simplest and most direct technique for analyzing the distribution course and branching patterns of segmental bronchi. Although it is thought to be the most precise approach for examining bronchial ramifications, the technique and process of creating a bronchial tree casting reveal significant restrictions and difficulties, such as the finite number of specimens and the demand for contrasting hues (6,11). Bronchography is an additional method that can be utilized for the purpose of investigating the anatomical structure of a living human individual. However, the current technology solely provides 2D images, which may lack precision as a result of the overlapping of bronchi in 3D space (12,25). While CT scanning, notably transverse CT and spiral CT, has the potential to yield superior imaging outcomes, its implementation can be challenging due to the need for spatial integration of quantitative data obtained from various sections (10,12,13). Finally, fiberoptic bronchoscopy is an invasive procedure that can only offer endobronchial perspectives, which may result in risks of respiratory irritation, hemorrhage, infection, and the rare occurrence of lung collapse (8).
The current study employed the Siemens syngo.via post-processing workstation to generate 3D reconstructions of bronchial structure and VB perspectives. This allowed for the display of both extrabronchial and endobronchial views of the bronchial tree, making it easy to interpret bronchial branching configurations (25,26). In addition, the 3D spatial adjustment facilitated the representation of composite bronchial structures with improved anatomical accuracy, thereby addressing the limitations associated with the overlapping and unclear visualization of segmental bronchi encountered in 2D techniques like bronchography and transverse CT (6,19,25,27). Moreover, the advent of multi-detector CT has facilitated the acquisition of isotropic data during routine thorax examinations, simplifying the process of generating 3D and VB images compared to the construction of anatomical specimens.
In contrast to previous studies, the present investigation has improved the quality of the data by increasing the sample size and offering more accurate information regarding the bronchosegmental branching patterns (Table 3). Furthermore, the application of 3D and VB images facilitated the researchers to obtain a more comprehensive dataset regarding the diverse branching patterns and gender-related differences observed in the segmental bronchi. The findings of our research conducted on the RIL revealed mainly four anatomical types of bronchial tree, with type 1 being the most prevalent at 71.44%, while the remaining 16.06%, 7.40%, and 5.10% were classified as type 2, type 3, and type 4, respectively. Naidich et al. found that type 1 was present in 67.74% of cases, followed by type 2 (3.23%) and type 3 (9.68%) (13). Huang et al. reported 56.7% of the cases with type 1, 10.5% with type 2, and 8.82% were identified as having type 4 (25). There was a slight variation in the branching types and their ratios among these studies (Table 3).
Table 3
| Study | Research methods | Sample size | Right inferior lobe, % | Left inferior lobe, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | Type 4 | Type 1 | Type 2 | Type 3 | Type 4 | ||||
| Pitel 1953, (11) | Dissection | 50 | / | / | / | / | 56 | 30 | – | – | |
| Naidich 1988, (13) | Thin slice continuous CT | 31 | 67.74 | 3.23 | 9.68 | – | 66.67 | 6.67 | – | – | |
| Ma 1997, (28) | Fiberoptic bronchoscopy | 467 | / | / | / | / | 54.4 | – | – | – | |
| Zhao 2009, (6) | Spiral CT | 216 | / | / | / | / | 75 | 18 | – | – | |
| Huang 2019, (25) | Spiral CT | 238 | 56.7 | 10.5 | – | 8.82 | / | / | / | / | |
| Current study 2019–2021 | Somatom Definition Flash CT | 10,000 | 71.44 | 16.06 | 7.40 | 5.10 | 82.89 | 13.53 | 2.88 | 0.70 | |
– denotes ‘‘not found’’; / denotes ‘‘lung segment not included’’. CT, computerized tomography.
The current investigation on the LIL explored four anatomical types of bronchial tree, where type 1 was noted to be the most common branching type with 82.89%. This finding is consistent with earlier studies conducted by various researchers, who reported a frequency of 54.4% to 75% (Table 3). It was also discovered that the incidence of type 2 in the LIL was 13.53%, while earlier studies reported a frequency of 6.67% to 30% (Table 3). Additionally, we identified types 3 and 4 in the LIL, which had not been previously reported. This represents a significant variation in the bronchial branching types as compared to the previous studies. Hence, a comprehensive analysis that compares the findings of the current study with those of previous ones is presented in Table 3 (6,9,11,25,28).
Various research studies mentioned above have reported disparities in results regarding branching types, possibly due to variations in methodology and sample size. The current study analysed a substantial amount of data and offered a clear advantage by providing panoramic views of 3D bronchial tree images for accurate interpretation. Likewise, this research was the initial one to compare male and female categories to investigate any possible variations in bronchial branches. The results indicated significant differences between sexes regarding the proportion of bronchial branches in the RIL (P<0.05) (Table 1). Our description of the various anatomical patterns of the segmental bronchi in the bilateral inferior lobes can also aid in the detection and exact localization of any focal lesions in the lungs by identifying each bronchopulmonary segment (8,29,30). Having a thorough understanding of the bronchosegmental anatomy prior to surgery might be beneficial for surgeons when carrying out treatments such as sublobar resection or pulmonary segmentectomy. It also possesses the capability to provide accurate guidelines for the implementation of fiberoptic bronchoscopies (31-34). Without sufficient knowledge, the application of thoracic surgery or fiberoptic bronchoscopy can lead to inadequate treatment or failure. In relation to the applications of our investigation, it is worth noting that it holds potential benefits for COVID-19 patients who frequently necessitate non-invasive examinations to attain precise imaging diagnoses, thereby facilitating the precise and efficient implementation of medical procedures such as BAL, segmentectomy, lobectomy, and other similar approaches (18,20,35).
The limitations of this study include: First, this was a single-center, retrospective study, which may be with possible selection bias. Second, only Chinese people were chosen as research subjects. Finally, the selection of subjects was limited to individuals with pre-existing airway disorders as opposed to individuals who were in good condition.
The results may be more convincing if we recruit healthy volunteers as study subjects, but this will lose the advantage of a large sample size. However, this gives us a new idea, for instance, to investigate whether there is a statistical difference in the proportion of bronchial typing between a certain group of people (such as lung cancer patients) and healthy people. In addition to the large sample size, this study also adopted a research mode that integrated VR and VB. The implementation of VB allowed for enhanced identification of bronchial patterns that may otherwise be difficult to detect in VR images, hence augmenting the precision of the findings.
Conclusions
In conclusion, our research has effectively identified various bronchial branching patterns in the right and LILs using MSCT with 3D and VB images. Additionally, we have explored how these patterns can differ between sexes by analyzing a large dataset. Consequently, the outcomes of this research will offer significant knowledge for healthcare practitioners in planning surgical and interventional procedures. This will enable them to enhance their comprehension of the course and dispersion patterns of pulmonary segmental bronchial branches, including any potential variations.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-213/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-213/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Ethics Committee of the School of Basic Medicine of Shandong University (No. ECSBMSSDU2018-1-050). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mehran RJ. Fundamental and Practical Aspects of Airway Anatomy: From Glottis to Segmental Bronchus. Thorac Surg Clin 2018;28:117-25. [Crossref] [PubMed]
- Krause GR, Lubert M. The anatomy of the bronchopulmonary segments: clinical applications. Radiology 1951;56:333-54. [Crossref] [PubMed]
- NOMENCLATURE of broncho-pulmonary anatomy; an international nomenclature accepted by the Thoracic Society. Thorax 1950;5:222-8. [Crossref] [PubMed]
- Boyden EA. Segmental anatomy of the lungs a study of the patterns of the segmental bronchi and related pulmonary vessels. New York: McGraw-Hill, 1955.
- Chassagnon G, Morel B, Carpentier E, Ducou Le Pointe H, Sirinelli D. Tracheobronchial Branching Abnormalities: Lobe-based Classification Scheme. Radiographics 2016;36:358-73. [Crossref] [PubMed]
- Zhao X, Ju Y, Liu C, Li J, Huang M, Sun J, Wang T. Bronchial anatomy of left lung: a study of multi-detector row CT. Surg Radiol Anat 2009;31:85-91. [Crossref] [PubMed]
- D'Antoni AV. Gray's Anatomy, the Anatomical Basis of Clinical Practice, 41st edition. Clin Anat 2016;29:264-5.
- Beder S, Küpeli E, Karnak D, Kayacan O. Tracheobronchial variations in Turkish population. Clin Anat 2008;21:531-8. [Crossref] [PubMed]
- Ghaye B, Szapiro D, Fanchamps JM, Dondelinger RF. Congenital bronchial abnormalities revisited. Radiographics 2001;21:105-19. [Crossref] [PubMed]
- Mori K, Ema S, Kitasaka T, Mekada Y, Ide I, Murase H, Suenaga Y, Takabatake H, Mori M, Natori H. Automated nomenclature of bronchial branches extracted from CT images and its application to biopsy path planning in virtual bronchoscopy. Med Image Comput Comput Assist Interv 2005;8:854-61.
- Pitel M, Boyden EA. Variations in the bronchovascular patterns of the left lower lobe of fifty lungs. J Thorac Surg 1953;26:633-53.
- Le Roux BT. The bronchial anatomy of the left upper lobe. J Thorac Cardiovasc Surg 1962;44:216-24.
- Naidich DP, Zinn WL, Ettenger NA, McCauley DI, Garay SM. Basilar segmental bronchi: thin-section CT evaluation. Radiology 1988;169:11-6. [Crossref] [PubMed]
- Lee KS, Bae WK, Lee BH, Kim IY, Choi EW, Lee BH. Bronchovascular anatomy of the upper lobes: evaluation with thin-section CT. Radiology 1991;181:765-72. [Crossref] [PubMed]
- Sealy WC, Connally SR, Dalton ML. Naming the bronchopulmonary segments and the development of pulmonary surgery. Ann Thorac Surg 1993;55:184-8. [Crossref] [PubMed]
- Hammond E, Chan KS, Ames JC, Stoyles N, Sloan CM, Guo J, Newell JD Jr, Hoffman EA, Sieren JC. Impact of advanced detector technology and iterative reconstruction on low-dose quantitative assessment of lung computed tomography density in a biological lung model. Med Phys 2018; Epub ahead of print. [Crossref]
- Dalrymple NC, Prasad SR, Freckleton MW, Chintapalli KN. Informatics in radiology (infoRAD): introduction to the language of three-dimensional imaging with multidetector CT. Radiographics 2005;25:1409-28. [Crossref] [PubMed]
- Horton KM, Horton MR, Fishman EK. Advanced visualization of airways with 64-MDCT: 3D mapping and virtual bronchoscopy. AJR Am J Roentgenol 2007;189:1387-96. [Crossref] [PubMed]
- Kiraly AP, Helferty JP, Hoffman EA, McLennan G, Higgins WE. Three-dimensional path planning for virtual bronchoscopy. IEEE Trans Med Imaging 2004;23:1365-79. [Crossref] [PubMed]
- Rosell J, Cabras P. A three-stage method for the 3D reconstruction of the tracheobronchial tree from CT scans. Comput Med Imaging Graph 2013;37:430-7. [Crossref] [PubMed]
- Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am J Roentgenol 2020;214:1280-6. [Crossref] [PubMed]
- Wahidi MM, Shojaee S, Lamb CR, Ost D, Maldonado F, Eapen G, Caroff DA, Stevens MP, Ouellette DR, Lilly C, Gardner DD, Glisinski K, Pennington K, Alalawi R. The Use of Bronchoscopy During the Coronavirus Disease 2019 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. Chest 2020;158:1268-81. [Crossref] [PubMed]
- Scannell JG. Bronchographic anatomy of the lungs. Surg Clin North Am 1949;29:573-81. [Crossref] [PubMed]
- Perhomaa M, Lähde S, Rossi O, Suramo I. Helical CT in evaluation of the bronchial tree. Acta Radiol 1997;38:83-91. [Crossref] [PubMed]
- Huang M, Wang T, Wang X, Zhao X. An anatomical study of the right bronchial tree using multi-detector computed tomography. Surg Radiol Anat 2019;41:335-8. [Crossref] [PubMed]
- Wang T, Meng M, Huang M, Zhao X. Variations of right bronchial tree: a study with multi-detector CT. Surg Radiol Anat 2018;40:955-8. [Crossref] [PubMed]
- Gariani J, Martin SP, Botsikas D, Becker CD, Montet X. Evaluating the effect of increased pitch, iterative reconstruction and dual source CT on dose reduction and image quality. Br J Radiol 2018;91:20170443. [Crossref] [PubMed]
- Ma X, Ma Y, Wang S. Analysis of 270 cases of bronchial variation in lung segment. Chinese Journal of Practical Internal Medicine 1997;22.
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. [Crossref] [PubMed]
- Salvolini L, Bichi Secchi E, Costarelli L, De Nicola M. Clinical applications of 2D and 3D CT imaging of the airways--a review. Eur J Radiol 2000;34:9-25. [Crossref] [PubMed]
- Yang Q, Xie B, Hu M, Sun X, Huang X, Guo M. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, Xu J, Zhu Q, Chen L. Thoracoscopic Pulmonary Sub-Subsegmentectomy Based on Three-Dimensional Images. Ann Thorac Surg 2016;102:e389-91. [Crossref] [PubMed]
- Ugalde P, Camargo Jde J, Deslauriers J. Lobes, fissures, and bronchopulmonary segments. Thorac Surg Clin 2007;17:587-99. [Crossref] [PubMed]
- Fetita CI, Prêteux F, Beigelman-Aubry C, Grenier P. Pulmonary airways: 3-D reconstruction from multislice CT and clinical investigation. IEEE Trans Med Imaging 2004;23:1353-64. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, Singh D, Macherey R, Bartley S, Santucci T. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]


