
Ten-year experiences and outcomes of bypass surgery and endovascular therapy in the management of infrarenal aortic occlusion: a single-center retrospective cohort study
Introduction
Infrarenal aortic occlusion (IAO) is a complete occlusion of infrarenal aorta classified as TransAtlantic Inter-Society Consensus (TASC II) Type D lesions (1). It is the most complicated and severe subclass of aortoiliac occlusive disease (AIOD). Symptoms and signs of IAO include loss of femoral artery beats, intermittent claudication, and erectile dysfunction in male patients (2). The incidence of IAO is low, with a prevalence of 0.15% and accounting for only 3–8.5% of AIOD (3,4). Although the TASC II guidelines recommend open reconstructive surgery for IAO as the first-line treatment (II C), its perioperative mortality rate can be 3–4% (2). In recent years, with the advancement of endovascular techniques, the indications for endovascular treatment (EVT) of complex aortoiliac lesions have been gradually expanded (5-9), and the European Society for Vascular Surgery (ESVS) has proposed that EVT can be the first-line treatment for severe AIOD cases that cannot tolerate open surgery (10).
Although EVT has shown promising potential in the treatment of IAO lesions, only a few studies have directly compared the safety and patency of bypass surgery with EVT (11,12), and it remains unknown whether the safety and efficacy of EVT for IAO are comparable to bypass surgery. Therefore, a retrospective study was conducted to compare the clinical outcomes of bypass surgery and EVT in real-world clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-236/rc).
Methods
Patients and study cohort
Based on practical consideration, a retrospective review was conducted for 92 patients hospitalized at Peking Union Medical College Hospital for surgical treatment of IAO between January 2011 and December 2021 (Figure 1). The Institutional Review Board of Peking Union Medical College Hospital approved this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study.
All patients in this study had symptoms of intermittent claudication and were graded primarily according to the Rutherford classification and ankle-brachial index (ABI). The diagnosis was confirmed by computed tomography angiography (CTA) for all patients. This study included consecutive patients with chronic (duration >2 weeks) and atherosclerotic total IAO (involvement of the aortic bifurcation with bilateral iliac arteries). It was ensured that the distal outflow track stenosis was less than 30%, and patients with at least one vessel flowing to the foot and limb ischemia and rheumatic arterial disease caused aortic occlusion or acute thrombosis only were excluded.
The surgical options available to patients were determined by their vascular anatomy and health status, with a focus on informing them on the differences between bypass surgery and EVT. Patients with few comorbidities, complex lesions and younger age were generally advised to undergo open surgery, as well as for patients with juxtarenal occlusions and heavy vascular calcification that made interventional treatment difficult. Additionally, factors such as trauma, cost, and patient preference were taken into account in surgical procedure management. However, with the increasing experience of surgeons and the development of interventional techniques, EVT became the preferred first-line treatment. It is worth noting that two patients were selected for open surgery after the failure of EVT, and this subset of patients was excluded from the EVT group.
Treatment procedures
EVT
All endovascular cases were performed under local anesthesia by a surgical team. The left brachial artery was punctured, and a catheter combined with a guidewire passed through the aortic arch and the descending aorta to the abdominal aorta. Then the surgeon replaced the catheter with a 6/7F 90-cm long sheath. The catheter, along with the 0.035 or 0.018 guidewires, was delivered through the occlusive segments to the true lumen of the femoral artery. The femoral arteries were punctured, a short sheath implanted, and the working guidewire reestablished through the sheath side by side. The surgeon pre-dilated the occlusion segments of the abdominal aorta and the iliac artery using a balloon (e.g., Reekross 4 mm × 120 mm, Bard, Murray Hill, NJ, USA), and implanted two stents (e.g., Medtronic, 8 mm × 100 mm, 8 mm × 120 mm, Minneapolis, MN, USA) along with the guidewire by kissing technique. If the lesion was not covered completely, a stent of suitable length was implanted jointly. Then the occlusive part was expanded again inside the stents with a balloon (e.g., ADMIRAL 6 mm × 80 mm or longer, Medtronic). The specific stent type depended on the surgeon’s preference and the lesion. After that, the surgeon performed angiography to confirm blood flow and apposition of the stents. All patients who underwent EVT or bypass received dual antiplatelet therapy (aspirin 100 mg/d and clopidogrel 75 mg/d) for at least 6 months and then switched to single antiplatelet therapy.
Bypass surgery
Surgeries were performed under general anesthesia using a transperitoneal midline incision and longitudinal inguinal incision to expose the abdominal aorta and the femoral arteries, respectively. After heparinization, the surgeon clamped the abdominal aorta at a suitable level according to the upper edge of the occlusion. If renal arteries were involved, then they needed to be clamped as well. The surgeon cut the abdominal aorta, 2 cm below the level of the renal artery and then stripped out the thrombus. A bifurcated artificial graft (GORE-TEX 16-8 mm, W.L. Gore & Associates, Inc., Newark, DE, USA) was employed retroperitoneally, and end-to-side anastomoses at the aorta and femoral artery were performed with Prolene 3-0 or 5-0, respectively. Another option for patients with difficulty in standard bypass surgery is the extra-anatomic revascularization approach, mainly axillo-femoral bypass (AxFB) where all anastomoses are performed in a termino-lateral fashion.
Technical success rate was evaluated by digital subtraction angiography (residual stenosis was less than 30%) (13). The arterial pulsation of lower extremities was evaluated immediately after operation. The Rutherford classification of both lower limbs was evaluated within 30 days after operation. Perioperative complications included any complication requiring additional treatment or prolongation of the hospital stay. Severe perioperative complications include life-threatening hemorrhage, severe infection, acute renal injury and cardiovascular events (including atrial fibrillation, myocardial infarction, etc.).
Follow-up
All cases were followed up at 1, 6, and 12 months postoperatively and every 6 or 12 months after that; follow-up was defined as the time from surgery to the last outpatient visit. Review assessments included improvement in symptoms, palpable arterial pulsations, and noninvasive vascular examinations (Doppler ultrasound, ABI or CTA). Technical success was defined as revascularization of the occluded artery, and clinical success was defined as Rutherford classification returned to class 0 (14). The surgeon reevaluated the symptoms, artery pulses of the lower limb, and ABI. For patients with aggravation of claudication, loss of previous palpable pulse, and decrease in ABI (>0.15) compared to the best post-operative condition, another intervention could be considered after further examination.
Outcome measures
The primary endpoint was the primary patency. Secondary endpoints included technical success, procedural mortality, length of hospital stay, severe complications, postoperative Rutherford classification improvement, secondary patency and overall survival. Primary patency was defined as the interval from the establishment of the access to the time of any intervention aimed at maintaining or re-establishing patency or measuring patency. Secondary patency was defined as the interval from the placement of the access to the abandonment of the access or measurement of patency, including interventions aimed at re-establishing or maintaining access function (surgical or endovascular interventions). Overall survival was defined as the interval from the establishment of the access to the time of any intervention aimed at maintaining or re-establishing patency or measuring patency or loss of follow-up.
Data analyses
Quantitative data were described as mean ± standard error of mean (SEM) or mean (range). Categorical variables were expressed as frequencies (percentages) and compared by Chi-squared test. Differences between groups were tested using Welch’s t-test for continuous variables. Ordered categorical data were compared using the rank-sum test. The curve of the accumulative probability of survival was determined by the Kaplan-Meier survival analysis (Mantel-Cox test). Patients who were lost during follow-up were counted as event occurrences in overall survival analyses. Patients with missing values were excluded from subsequent analyses. P values were all two-sided, and statistical significance was defined as P<0.050. All statistical analyses were performed by using GraphPad Prism (7.0 version, GraphPad Software Inc, San Diego, CA, USA).
Results
Demographics and preoperative comorbidities
Between January 2011 and December 2021, a total of 92 IAO patients were included in this research, with 40 patients receiving EVT and 52 patients receiving bypass surgery. The numbers of procedures performed during the study period are shown in Figure 2. The mean age (61.58 years for the EVT group, 57.98 years for the bypass group) and male proportion (90.00% for the EVT group, 94.23% for the bypass group) in the two groups were similar (P=0.059 and P=0.448, respectively). Cardiovascular risk factors, including hypertension, diabetes mellitus, coronary artery disease, hyperlipidemia, smoking, and hyperhomocysteinemia were equally prevalent between the two groups (all P>0.05). As for other comorbidities, 5 patients had renal insufficiency, and 17 patients had a cerebral infarction in total. Both were equally common between the two groups (P=0.872 and P=0.066, respectively) (Table 1).
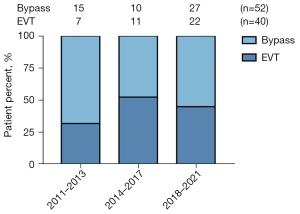
Table 1
| Variable | Total (n=92) | EVT (n=40) | Bypass (n=52) | P value |
|---|---|---|---|---|
| Mean age, years | 59.54 [37–87] | 61.58 [37–87] | 57.98 [42–77] | 0.059 |
| Male | 85 (92.39) | 36 (90.00) | 49 (94.23) | 0.448 |
| Hypertension | 54 (58.70) | 26 (65.00) | 28 (53.85) | 0.281 |
| Diabetes mellitus | 15 (16.30) | 4 (10.00) | 11 (21.15) | 0.151 |
| Coronary artery disease | 19 (20.65) | 10 (25.00) | 9 (17.31) | 0.366 |
| Renal insufficiency | 5 (5.43) | 2 (5.00) | 3 (5.77) | 0.872 |
| Cerebral infarction | 17 (18.48) | 4 (10.00) | 13 (25.00) | 0.066 |
| Hyperlipidemia | 30 (32.61) | 13 (32.50) | 17 (32.69) | 0.984 |
| Smoking | 80 (86.96) | 34 (85.00) | 46 (88.46) | 0.625 |
| Smoking index† | 703.5±54.14 | 645±72.03 | 749.2±78.17 | 0.329 |
| Hyperhomocysteinemia | 14 (15.22) | 6 (15.00) | 8 (15.38) | 0.887 |
Data are presented as mean [range], n (%), and mean ± standard error of mean. †, smoking index = years of smoking × number of cigarettes per day, the unit is package year. EVT, endovascular treatment.
Preoperative Rutherford classification and physical examination
Most patients had severe claudication. According to the Rutherford classification, 12.0% of patients (n=11) were class II, 63.0% (n=58) were class III, 14.1% (n=13) were class IV, 6.5% (n=6) were class V, 3.3% (n=3) were class VI. Preoperative physical examination showed that most patients had diminished arterial pulse and a significant decrease in ABI (left side: total 0.16±0.03, EVT 0.23±0.06, bypass 0.11±0.03; right side: total 0.16±0.03, EVT 0.23±0.06, bypass 0.11±0.03) (Table 2). The two groups were matched in Rutherford classification (P=0.464) and ABI (P>0.05; left side and right side, P=0.097 and P=0.088, respectively) (Table 2).
Table 2
| Variable | Total (n=92) | EVT (n=40) | Bypass (n=52) | P value |
|---|---|---|---|---|
| Rutherford classification | 0.464 | |||
| I | 1 | 1 | 0 | |
| II | 11 | 5 | 6 | |
| III | 58 | 25 | 33 | |
| IV | 13 | 5 | 8 | |
| V | 6 | 4 | 2 | |
| VI | 3 | 0 | 3 | |
| Artery pulse | ||||
| Left femoral | 25.00% (23/92) | 32.50% (13/40) | 19.23% (10/52) | 0.145 |
| Left popliteal | 13.64% (12/88) | 15.38% (6/39) | 12.24% (6/49) | 0.670 |
| Left posterior tibial | 8.99% (8/89) | 12.82% (5/39) | 6.00% (3/50) | 0.264 |
| Left dorsal | 12.79% (11/86) | 12.50% (5/40) | 13.04% (6/46) | 0.888 |
| Right femoral | 20.65% (19/92) | 25.00% (10/40) | 17.31% (9/52) | 0.366 |
| Right popliteal | 12.50% (11/88) | 10.26% (4/39) | 14.29% (7/49) | 0.570 |
| Right posterior tibial | 10.11% (9/89) | 10.26% (4/39) | 10.00% (5/50) | 0.968 |
| Right dorsal | 13.04% (12/92) | 15.00% (6/40) | 11.54% (6/52) | 0.625 |
| Left ABI | 0.16±0.03 | 0.23±0.06 | 0.11±0.03 | 0.097 |
| Right ABI | 0.16±0.03 | 0.23±0.06 | 0.11±0.03 | 0.088 |
Data are presented as number, percentage, and mean ± standard error of mean. EVT, endovascular treatment; ABI, ankle-brachial index.
Perioperative period
Revascularization was all achieved in the aortofemoral bypass (AoFB) group (100%, n=52). In the EVT group, there was one case of technical failure, and the technical success rate was 97.5% (39/40). One case was that the guidewire could not pass through the occluded segment to establish a working route. Considering the patient’s advanced age (>80 years old) and cardiac dysfunction (ejection fraction 39%), the surgeon did not perform further treatment. One failed EVT case was not accounted into the calculation of postoperative Rutherford classification. There was no statistical difference in the technical success rate between the two groups (P=0.252). The one severe complication in the EVT group was a retroperitoneal hematoma; the six severe complications of the AoFB group were severe pneumonia, infection of grafts, severe acute coronary syndrome, acute anterior myocardial infarction combined with pneumonia, gastrointestinal hemorrhage, and acute renal infarction, respectively.
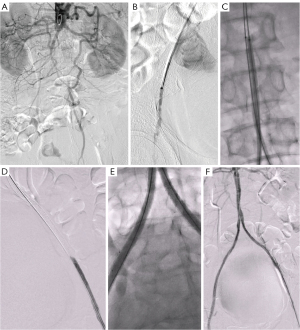
The proportion of serious complications in EVT, the group was less than that of the bypass group [2.50% (1/40) in the EVT group, 11.54% (6/52) in the AoFB group], but there was no statistical difference due to the small sample size (Table 3). A representative example of the EVT procedure is shown in Figure 3. The duration of hospital stays was 5.15±0.72 days for the EVT group and 11.83±0.67 days for the bypass group (Table 3). There was a significant difference in hospital stay between the EVT and bypass groups (P<0.0001).
Table 3
| Variable | Total (n=92) | EVT (n=40) | Bypass (n=52) | P value |
|---|---|---|---|---|
| Technical success rate | 91 (98.91) | 39 (97.50) | 52 (100.00) | 0.252 |
| No. of complications | 11 (11.96) | 3 (7.50) | 8 (15.38) | 0.248 |
| No. of severe complications† | 7 (7.61) | 1 (2.50) | 6 (11.54) | 0.064 |
| Length of stay (day) | 8.97±0.60 | 5.15±0.72 | 11.83±0.67 | <0.0001 |
| No. of effective follow-up | 83 (90.22) | 37 (92.50) | 46 (88.46) | 0.518 |
| Follow-up (month) | 41.04±3.13 | 34.54±4.36 | 46.38±4.33 | 0.597 |
| Postoperative Rutherford classification‡ | n=77 | n=34 | n=43 | 0.988 |
| Asymptomatic (0) | 70 | 31 | 39 | |
| I | 1 | 0 | 1 | |
| II | 1 | 1 | 0 | |
| III | 5 | 2 | 3 | |
| IV | 0 | 0 | 0 | |
| V | 0 | 0 | 0 | |
| VI | 0 | 0 | 0 | |
| Clinical success rate | 90.91% | 91.18% | 90.70% | 0.942 |
†, definition of severe complications: life-threatening bleeding, infection, or acute coronary disease. The 1 severe complication in the EVT group: retroperitoneal hematoma, respectively; the 6 severe complications of bypass group: severe pneumonia, infection of grafts, severe acute coronary syndrome, acute anterior myocardial infarction combined with pneumonia, gastrointestinal hemorrhage and acute renal infarction, respectively. ‡, postoperative Rutherford classification was performed within 2 weeks after surgery. Patients who lost follow-up after operation and were not suitable for Rutherford classification after operation were excluded. Data are presented as n (%), mean ± standard error of mean, and number unless otherwise indicated. EVT, endovascular treatment.
Procedural details
In the bypass surgery group, a total of 41 (78.85%) underwent AoFB, 11 (21.15%) underwent AxFB, and 3 patients underwent embolectomy of the femoral and popliteal arteries for distal arterial embolism. In the EVT group, the average number of stents implanted per limb was 1.720±0.051, the average length of stents was 111.6±4.051 mm, and the average width of stents was 7.706±0.075 mm. In 39 patients, 110 bare-metal stents (90.91%) and 11 covered stents (9.09%) were implanted, including kissing stent technique in 27 patients (69.23%), non-kissing stent technique in 12 patients (30.77%) and covered endovascular reconstruction of aortic bifurcation (CERAB) technique in 2 patients (5.13%) (Table 4).
Table 4
| Variable | Bypass | EVT |
|---|---|---|
| Axillofemoral bypass | 11 (21.15) | – |
| Aortofemoral bypass | 41 (78.85) | – |
| Aortic stents | ||
| Non-kissing stents | – | 12 (30.77) |
| Kissing stents | – | 27 (69.23) |
| CREAB technique | – | 2 (5.13) |
| Iliac stents | ||
| Bare-metal stent | – | 110 (90.91) |
| Covered stent | – | 11 (9.09) |
| Number of stents (per limb) | – | 1.720±0.051 [1–3] |
| Mean length (mm) | – | 111.6±4.051 [40–200] |
| Mean diameter (mm) | – | 7.706±0.075 [6–10] |
| Catheter-directed thrombolysis | – | 9 (23.08) |
| Femoral endarterectomy | – | 4 (10.26) |
Data are presented as n (%) and mean ± standard error of mean [range]. EVT, endovascular treatment; CREAB, covered endovascular reconstruction of aortic bifurcation.
Follow-up
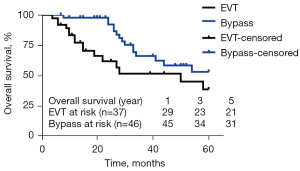
Eighty-three out of 92 patients had follow-up after surgery. The mean follow-up period was 41.04±3.13 months. Seventy out of the 92 patients’ symptoms disappeared after the operation and reached an asymptomatic state (Table 3). The 1-, 3-, and 5-year primary patency rates were 81.8%, 73.1%, and 73.1% in the EVT group and 97.8%, 80.6%, and 80.6% in the bypass group. One-year primary patency rates were lower in the EVT group than in the bypass group (81.8% vs. 97.8%; P=0.016; Figure 4A). Five-year primary patency rates presented no difference between the two groups (80.6% vs. 73.1%; P=0.456; Figure 4A). It is suggested that the difference in primary patency between endovascular therapy and bypass therapy is mainly reflected in the first year after surgery, which may be closely related to intimal hyperplasia mediated by vascular implants, smoking status and hyperlipidemia. Life management after endovascular therapy should be emphasized and patients should be strictly followed up. There was a significant difference in the secondary patency rate (100% vs. 81.6%; P=0.005; Figure 4B). Overall survival rates were higher in the bypass surgery group than in the EVT group, at 1 year (97.7% vs. 83.4%), 3 years (66.2% vs. 51.6%) and 5 years (53.1% vs. 38.7%) (P=0.035; Figure 5).

Discussion
Since the 1960s, AoFB has been the primary treatment for IAO. A review of the literature on the treatment of AIOD using three surgical procedures, AoFB, iliofemoral bypass (IFB), and aortoiliac endarterectomy (AIE), showed that the operative mortality for the three procedures was 4.1% for AoFB, 2.7% for IFB and AIE; the 5-year primary patency rates for AoFB, IFB, and AIE were 86.3%, 85.3%, and 88.3%, respectively (2). Surgical techniques have been developed to reduce ischemic damage to kidneys in proximal IAO (15). In our center, 39 of 52 patients returned to the asymptomatic state, with a 5-year primary patency rate of 80.6%. While no perioperative deaths occurred in our study, 11.54% (6/52) of patients experienced life-threatening complications, indicating the importance of comprehensive patient evaluation to determine eligibility for bypass surgery.
Compared with conventional open surgery, laparoscopic aorto-bifemoral bypass surgery has similar or fewer complications, shorter postoperative hospital stay, and lower costs (10,15,16). A retrospective cohort analysis demonstrated that the 5-year primary and secondary patency rates were as high as 83% and 97% (17), although more data are required to further support the findings (due to the limited number of reported cases, the sample size was small). However, the long learning curve of laparoscopic bypass surgery (18) has limited the number of centers performing this procedure.
With the rapid development of EVT, the TASC guideline has recommended EVT as the preferred treatment for short stenosis or short occlusion of the iliac artery segment (types A and B in the TASC grading) (1). However, treating IAO (type D) can be challenging due to problems in establishing guidewire pathways. Re-entry devices (16) and hybrid surgery can be used adjunctively to increase the success rate of establishing working pathways. In our EVT group (n=39), 4 patients performed femoral endarterectomy as adjunctive treatments (Table 4). For extensive stenosis or occlusion of the aortoiliac artery (TASC grading, types C and D), a recent meta-analysis showed that EVT also has a technical success rate of 90% and a one-year primary patency rate of nearly 90% (17). Cases of EVT for IAO have been reported in the early 1990s (18,19), and more case series studies have appeared in recent years (9,11,20-22). More recently, a case series study including 49 IAO patients treated with EVT reported an 88.4% primary patency at 1 year and 80.1% at 3 years (9), while another case series research including 31 IAO patients reported primary patency of 85% and 66% at 1 and 3 years, respectively (23). A midterm study comparing open surgery and EVT for IAO showed that the surgical group had higher primary patency rates at 1, 3, and 5 years than the EVT group, with no difference in secondary patency rates or survival between the two groups, but the EVT group had a shorter hospital stay and lower postoperative complication rates (11). A 10-year single-center study showed that the EVT group had the same primary patency as the bypass surgery group at 5 years, with the advantage of fewer surgical complications and shorter hospital stays, and could be considered as an effective procedure for AIOD patients with TASC-II C/D (7). A multicenter clinical registry study from Asia (CHAOS Registry) showed that bypass surgery still had significantly better primary patency rates compared to EVT, yet with no difference in secondary patency and reintervention rates at five years, and no difference in surgical success, complications, or mortality, with advantages only in operative time and hospital stay (13), similar to our results. As the growing data support the use of EVT for treating IAO, whether EVT is comparable to the first-line AoFB surgery requires to be answered.
Distal patency can be affected by multiple factors, such as distal outflow condition and stent type, etc. However, the AIOD can be relatively independent. In patients with combined common femoral artery bifurcation lesions, hybridization can be used to open the vessel while performing femoral endarterectomy and/or femoral-popliteal artery bypass grafting. Fifteen patients in this study underwent femoral endarterectomy simultaneously, while three patients underwent femoral-popliteal artery bypass grafting. To date, few comparative data are available to evaluate the effectiveness of the various types of stents. Typically, bare-metal stents, such as balloon-expandable stents and self-expanding stents are commonly used. Covered stents are generally accepted for patients with severe AIOD risk of thrombosis or rupture (24). However, they also risk obscuring collateral vessels or internal iliac arteries. A randomized controlled trial (COBEST) revealed that covered stents were more advantageous than bare-metal stents for long and short lesions with heavier calcification (25). In recent years, the covered endovascular reconstruction of the aortic bifurcation, known as CERAB, has been used for patients with extensive AIOD. Some studies have reported a 3-year primary patency rate of 82% with the CERAB technique (26), but further research is needed to investigate its long-term results.
This study was a retrospective study with limitations. The collection and follow-up data can be somewhat limited, and future randomized clinical trials with different surgical approaches or different types of implants need to be designed for the certainty of specific outcomes. The design of revascularization options is non-randomized and is left to the surgeon’s judgment based on the patient’s condition. The individual patient’s wishes were considered, which to some extent constituted a possible selection bias. However, the suitability of the patient’s lesion characteristics for endoluminal treatment is a factor to be considered, such as the presence of lesions in the contralateral iliac artery, the presence of arteries distal to the inguinal ligament, and the presence of the common femoral artery. The need for simultaneous treatment, the presence of common femoral artery lesions, the angulation of the bifurcation of the main iliac artery, whether the target lesion is a chronic completely occlusive lesion, and the availability of the brachial (radial) artery, should all be taken into account. In patients with long or complete bilateral iliac artery occlusion or failed access through bilateral femoral arteries, the operator can perform a retrograde puncture through the brachial artery of the upper extremity and guide the catheter into the descending aorta to the bifurcation of the abdominal aorta, which in our experience has shown that it is often easier to open the AIOD occluded lesion in these patients. In addition, for open surgery, it is also necessary to consider the case of lesion characteristics. Non-simple iliac artery lesions involving the abdominal aorta should be treated depending on the specific lesion location and degree (such as unilateral lesion, complete occlusion of the common iliac artery and external iliac artery bilaterally, or single/pure external iliac artery occlusion). The choice of bypass modality should be made accordingly, such as an anatomically oriented AoFB or iliac-femoral bypass, or an extra-anatomic. The bypass method of choice is either an anatomic aortofemoral or iliac-femoral bypass, or an extra-anatomic femoral-femoral bypass. It is important to note that the presence and extent of coexisting lesions in the common femoral artery often determine the extent of AIOD revascularization and the extent of the lesion often determine whether AIOD revascularization should be performed by bypass, endoluminal treatment or hybrid surgery. Finally, although the study involved clinical data spanning of 10 years, the overall number of cases was still small, which limited the statistical comparisons.
Conclusions
A 10-year real-world observative study was conducted in our center to compare the safety and efficacy of EVT and bypass surgery for IAO. The study showed that the application of EVT for IAO was safe, feasible and effective in the real world, with a 73.1% primary patency rate at 5 years. In addition, EVT was associated with significantly shorter hospital stays and fewer severe surgical complications than bypass surgery. Therefore, the safety and efficacy of EVT for IAO were comparable to that of bypass surgery, and EVT can be a viable option for treating patients with IAO.
Acknowledgments
Funding: This study received funding from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-236/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-236/coif). JS reports that National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-A-078) was received to support the study; ZL reports that National Natural Science Foundation of China (No. 82100521), National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-A-189) and Fundamental Research Funds for the Central Universities (No. 3332020009) were received to support the study; BL reports that National Natural Science Foundation of China (No. 82070498), CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2022-I2M-C&T-A-002) and Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2022-JKCS-09) were received to support the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Peking Union Medical College Hospital approved this study. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45 Suppl S:S5-67.
- Chiu KW, Davies RS, Nightingale PG, Bradbury AW, Adam DJ. Review of direct anatomical open surgical management of atherosclerotic aorto-iliac occlusive disease. Eur J Vasc Endovasc Surg 2010;39:460-71. [Crossref] [PubMed]
- Marrocco-Trischitta MM, Bertoglio L, Tshomba Y, Kahlberg A, Marone EM, Chiesa R. The best treatment of juxtarenal aortic occlusion is and will be open surgery. J Cardiovasc Surg (Torino) 2012;53:307-12.
- STARER F. SUTTON D. Aortic thrombosis. Br Med J 1958;1:1255-63. [Crossref] [PubMed]
- Soga Y, Iida O, Kawasaki D, Yamauchi Y, Suzuki K, Hirano K, Koshida R, Kamoi D, Tazaki J, Higashitani M, Shintani Y, Yamaoka T, Okazaki S, Suematsu N, Tsuchiya T, Miyashita Y, Shinozaki N, Takahashi HREAL-AI investigators. Contemporary outcomes after endovascular treatment for aorto-iliac artery disease. Circ J 2012;76:2697-704. [Crossref] [PubMed]
- Dorigo W, Piffaretti G, Benedetto F, Tarallo A, Castelli P, Spinelli F, Fargion A, Pratesi C. A comparison between aortobifemoral bypass and aortoiliac kissing stents in patients with complex aortoiliac obstructive disease. J Vasc Surg 2017;65:99-107. [Crossref] [PubMed]
- Squizzato F, D'Oria M, Bozza R, Porcellato L, Grego F, Lepidi S. Propensity-Matched Comparison of Endovascular versus Open Reconstruction for TASC-II C/D AortoIliac Occlusive Disease. A Ten-Year Single-Center Experience with Self-Expanding Covered Stents. Ann Vasc Surg 2021;71:84-95. [Crossref] [PubMed]
- Yuan L, Bao J, Zhao Z, Feng X, Lu Q, Jing Z. Endovascular therapy for long-segment atherosclerotic aortoiliac occlusion. J Vasc Surg 2014;59:663-8. [Crossref] [PubMed]
- Kim TH, Ko YG, Kim U, Kim JS, Choi D, Hong MK, Jang Y, Shim WH. Outcomes of endovascular treatment of chronic total occlusion of the infrarenal aorta. J Vasc Surg 2011;53:1542-9. [Crossref] [PubMed]
- Halliday A, Bax JJ. The 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration With the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:301-2. [Crossref] [PubMed]
- Lun Y, Zhang J, Wu X, Gang Q, Shen S, Jiang H, Duan Z, Xin S. Comparison of midterm outcomes between surgical treatment and endovascular reconstruction for chronic infrarenal aortoiliac occlusion. J Vasc Interv Radiol 2015;26:196-204. [Crossref] [PubMed]
- Kretschmann T, Usai MV, Taneva GT, Pitoulias GA, Torsello G, Donas KP. The role of open and endovascular treatment of patients with chronic aortoiliac Leriche syndrome. Vascular 2020;28:68-73. [Crossref] [PubMed]
- Fujimura N, Takahara M, Obara H, Ichihashi S, George RK, Igari K, Banno H, Hozawa K, Yamaoka T, Kian CJ, Tan JWH, Park K, Skyi PYC, Kato T, Kawarada O. Comparison of Aortobifemoral Bypass and Endovascular Treatment for Chronic Infrarenal Abdominal Aortic Occlusion From the CHAOS (CHronic Abdominal Aortic Occlusion, ASian Multicenter) Registry. J Endovasc Ther 2023;30:828-37. [Crossref] [PubMed]
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517-38. [Crossref] [PubMed]
- Pearce FB Jr, Yang S, Shi R, Tan TW, Zhang WW. Circumferential Aortic Endarterectomy Followed with Immediate Infrarenal Clamping Obviates Suprarenal Clamping for Juxtarenal Aortoiliac Occlusion. Ann Vasc Surg 2016;34:48-54. [Crossref] [PubMed]
- Varcoe RL, Nammuni I, Lennox AF, Walsh WR. Endovascular reconstruction of the occluded aortoiliac segment using "double-barrel" self-expanding stents and selective use of the Outback LTD catheter. J Endovasc Ther 2011;18:25-31. [Crossref] [PubMed]
- Ye W, Liu CW, Ricco JB, Mani K, Zeng R, Jiang J. Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions. J Vasc Surg 2011;53:1728-37. [Crossref] [PubMed]
- Diethrich EB. Endovascular techniques for abdominal aortic occlusions. Int Angiol 1993;12:270-80.
- Pilger E, Decrinis M, Stark G, Koch G, Obernosterer A, Tischler R, Lafer M, Doder A. Thrombolytic treatment and balloon angioplasty in chronic occlusion of the aortic bifurcation. Ann Intern Med 1994;120:40-4. [Crossref] [PubMed]
- Morisaki K, Yamaoka T, Iwasa K, Ohmine T. Outcomes of Endovascular Therapy for Infrarenal Aortic Occlusion of TASC II D Classification. Ann Vasc Surg 2017;43:203-9. [Crossref] [PubMed]
- Kasemi H, Marino M, Dionisi CP, Di Angelo CL, Fadda GF. Seven-Year Approach Evolution of the Aortoiliac Occlusive Disease Endovascular Treatment. Ann Vasc Surg 2016;30:277-85. [Crossref] [PubMed]
- Schmalstieg J, Zeller T, Tübler T, Sixt S, Schwencke C, Sandstede J, Krankenberg H. Long term data of endovascularly treated patients with severe and complex aortoiliac occlusive disease. J Cardiovasc Surg (Torino) 2012;53:291-300.
- Moise MA, Alvarez-Tostado JA, Clair DG, Greenberg RK, Lyden SP, Srivastava SD, Eagleton M, Sarac TS, Kashyap VS. Endovascular management of chronic infrarenal aortic occlusion. J Endovasc Ther 2009;16:84-92. [Crossref] [PubMed]
- Mwipatayi BP, Thomas S, Wong J, Temple SE, Vijayan V, Jackson M, Burrows SACovered Versus Balloon Expandable Stent Trial (COBEST) Co-investigators. A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease. J Vasc Surg 2011;54:1561-70. [Crossref] [PubMed]
- Piazza M, Squizzato F, Dall'Antonia A, Lepidi S, Menegolo M, Grego F, Antonello M. Editor's Choice - Outcomes of Self Expanding PTFE Covered Stent Versus Bare Metal Stent for Chronic Iliac Artery Occlusion in Matched Cohorts Using Propensity Score Modelling. Eur J Vasc Endovasc Surg 2017;54:177-85. [Crossref] [PubMed]
- Grimme FA, Goverde PC, Verbruggen PJ, Zeebregts CJ, Reijnen MM. Editor's Choice--First Results of the Covered Endovascular Reconstruction of the Aortic Bifurcation (CERAB) Technique for Aortoiliac Occlusive Disease. Eur J Vasc Endovasc Surg 2015;50:638-47. [Crossref] [PubMed]



