
Predictive value of clinical characteristics and baseline 18F-FDG PET/CT quantization parameters in primary adrenal diffuse large B-cell lymphoma: a preliminary study
Introduction
Diffuse large B-cell lymphoma (DLBCL), constituting around 30% of all newly diagnosed non-Hodgkin lymphomas (NHL), is the predominant subtype of aggressive NHL in adults, with an estimated annual incidence of 150,000 cases worldwide (1,2). DLBCL can manifest in various extra-nodal sites, including the gastrointestinal tract, skin, soft tissues, bone, genitourinary tract, and others. Among these, primary adrenal diffuse large B-cell lymphoma (PA-DLBCL) is a rare occurrence, comprising 3% of extra-nodal DLBCL cases (3,4). Until now, no more than 200 cases have been reported in the literature (5-7). PA-DLBCL was reported to frequently have clinicopathologic features, including elevated lactate dehydrogenase (LDH) levels, the presence of B symptoms, a non-germinal center B-cell-like (non-GCB) subtype, and Bcl-6 gene rearrangement (8). The prognosis of PA-DLBCL is notably poor, with a high risk of relapse, as evidenced by a 2-year overall survival (OS) rate of only 68.3% following the first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone regimen (R-CHOP) (3,9,10). Consequently, accurate prediction of patient prognosis prior to treatment initiation and timely adjustment of the treatment plan are of paramount importance. However, the identification of high-risk patients using existing prognostic scoring systems, such as the International Prognostic Index (IPI), is currently limited and hindered by a lack of comprehensive biological and imaging data (11-13).
Positron emission tomography/computed tomography (PET/CT) imaging techniques are typically performed before treatment initiation in patients with DLBCL, providing indispensable functional and metabolic information regarding lymphoma lesions. This imaging method serves multiple purposes, including staging, restaging, evaluating disease aggressiveness, and monitoring treatment response, and has been incorporated into the revised International Workshop Criteria (IWG) (1,14-25). PET/CT is included in the staging of lymphomas due to its higher sensitivity compared to CT, enabling a more accurate assessment of treatment response against a baseline (21). Exploring quantitative imaging parameters as potential prognosticators for assessing disease burden and response is crucial. The standardization of PET/CT application is mandatory for quantitative analysis and highly desirable for optimal clinical practice (26). Quantitative parameters derived from 18F-fluorodeoxyglucose (18F-FDG) PET, including maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), have demonstrated predictive value for assessing outcomes in patients with DLBCL (27-29). However, the prognostic utility of 18F-FDG PET/CT in PA-DLBCL remains unstudied.
Therefore, in this retrospective study, we reviewed the medical records of PA-DLBCL patients from two institutions, aiming to assess the potential value of 18F-FDG PET/CT and related factors in predicting prognosis. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-803/rc).
Methods
Patients
We retrospectively collected data from 594 patients diagnosed with DLBCL through pathological histological examination at Peking University First Hospital and the First Affiliated Hospital of Zhengzhou University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Peking University First Hospital and the First Affiliated Hospital of Zhengzhou University, and the requirement for individual consent for this retrospective analysis was waived. The data was collected from January 2014 to December 2022. As there is no unified guideline standard for PA-DLBCL, we developed inclusion criteria by combining the criteria adopted by most studies with the actual situation of the hospitals (5,30,31). The inclusion criteria were as follows: (I) pathological diagnosis of unilateral or bilateral PA-DLBCL (n=62); (II) no previous history of lymphoma at other sites (n=53); (III) if lymph node or organ involvement at other sites was present, lesions at the adrenal site were considered the main lesions (n=47); (IV) no abnormal blood cells were observed in routine blood or bone marrow examinations (n=45); (V) patients had complete clinical information (n=45); (VI) all patients underwent 18F-FDG PET/CT examination (n=40). The exclusion criteria were as follows: (I) patients who had received treatment (including chemotherapy, radiation therapy, surgery, etc.) before the PET examination (n=4); (II) patients with concomitant other malignant tumors (n=2); (III) patients lost to follow-up (n=4); and (IV) involvement of kidney or peripheral lymph nodes that were difficult to measure with 18F-FDG PET/CT parameters (n=6).
Clinical information of patients was collected based on inpatient medical records, including age, gender, B symptoms, adrenocorticotropic hormone (ATCH), LDH, β2-microglobulin, albumin (Alb), ferritin (Fe), blood calcium, Eastern Cooperative Oncology Group performance status (ECOG PS), IPI (11), Ann Arbor staging (32), number of involved organs, and Hans’ algorithm (33).
FDG PET/CT acquisition
Patients were instructed to abstain from smoking, alcohol, coffee, and tea for 24 hours before the examination and to avoid strenuous exercise. They were also required to fast for at least 6 hours prior to the examination in order to maintain their blood glucose below 11.1 mmol/L. The 18F-FDG PET/CT scan [utilizing Philips GXL-16 PET/CT (Philips Healthcare, Chicago, IL, USA) and Siemens Biograph Truepoint 64 PET/CT (Siemens, Erlangen, Germany) machines] was performed 60–80 minutes after intravenous administration of 18F-FDG (3.70–5.18 MBq/kg, radiochemical purity >95%). Additionally, patients were instructed to consume 500–1,000 mL of water and empty their bladders after 60 minutes of quiet rest. Routine scans were conducted from the head to mid-thigh, with separate acquisitions of the head and torso. Spiral CT scans were initially performed using a tube voltage of 120 kV and a tube current of 100 mA, followed by reconstruction using a soft tissue algorithm with a layer thickness of 5 mm. PET scans were generally acquired in 7–10 beds, with each bed lasting 1.5–2.5 minutes. The PET images were attenuation-corrected using CT data and reconstructed through an iterative method for image reconstruction.
PET/CT image analysis
Transverse, sagittal, and coronal FDG PET/CT images were reviewed. Consensus on the presence of abnormal lesions was reached between two nuclear medicine physicians with 4 and 8 years of experience, respectively. The workstation automatically delineated the region of interest (ROI) for each patient’s adrenal lesion, utilizing a threshold of 41% SUVmax (34,35). Manual adjustments were made to exclude physiological uptake from adjacent tissues at each level, and the software automatically calculated CT attenuation value, SUVmax, SUVmean, MTV, and TLG for the lesion.
Follow-up
Follow-up visits are conducted utilizing a combination of telephone communication and inpatient medical records, with a frequency of once every 3 months during the follow-up period. The follow-up deadline was January 2023. OS was defined as the time of diagnosis to death or follow-up cut-off time of the patient.
Statistical analysis
Statistical analysis was performed using IBM SPSS 24.0 software (IBM Corp., Armonk, NY, USA). The Mann-Whitney U test, independent samples t-test, and chi-square test were used for univariate analyses under the appropriate circumstances. Quantitative data following a normal distribution were expressed as , whereas quantitative data with a non-normal distribution were expressed as M (Q1, Q3). Prognostic analysis of OS was conducted utilizing Kaplan-Meier survival analysis, as well as univariate and multifactor Cox proportional risk regression models. A statistically significant difference was defined as P<0.1 in the one-way analysis of factors and P<0.05 in the multifactorial analysis.
Results
Patient characteristics
The 24 enrolled patients comprised 16 men and 8 women (median age 65 years, range, 51–90 years) (Figure 1). Among them, lymph node involvement was observed in 18 patients, skeletal involvement in 14 patients, renal involvement in 5 patients, and central nervous system (CNS) involvement as well as intestinal involvement in 4 patients each. Additionally, there were three patients each with spleen involvement and lung involvement, and two patients each with cardiac involvement and reproductive involvement. There was one patient with pleural and pancreatic involvement (Figure 2). A total of 18 patients received 4–8 cycles of R-CHOP or a standard CHOP regimen as first-line treatment, high-dose methotrexate-based regimen during chemotherapy in 3 patients with CNS involvement, whereas 1 patient with CNS involvement achieved remission after undergoing six cycles of chemotherapy followed by hematopoietic stem cell transplantation (HSCT). There were six patients who did not receive any treatment. Glucocorticoids were administered to patients with adrenocortical insufficiency before chemotherapy regimen. The median follow-up period was 17.5 months (range, 1–107 months). At the cut-off of follow-up, 16 cases had died. Among them, 15 patients died from disease progression, and 1 patient died due to a lung infection 1 year after autologous HSCT. The clinical data and baseline PET/CT parameters are summarized in Table 1.
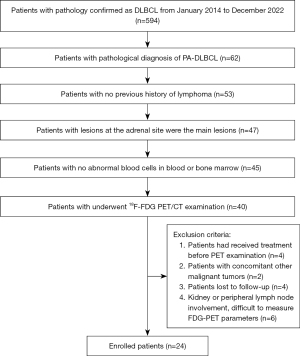

Table 1
| Characteristics | Values |
|---|---|
| Age (years) | |
| >60 | 18 (75.00) |
| ≤60 | 6 (25.00) |
| Sex | |
| Male | 16 (66.67) |
| Female | 8 (33.33) |
| Clinical symptoms | |
| Asymptomatic | 3 (12.50) |
| B symptom | 14 (58.33) |
| Abdominal pain | 7 (29.17) |
| Ann Arbor staging | |
| III/IV | 18 (75.00) |
| I/II | 6 (25.00) |
| ECOG PS | |
| 2/3/4 | 8 (33.33) |
| 0/1 | 16 (66.67) |
| IPI | |
| 2/3/4 | 13 (54.17) |
| 0/1 | 11 (45.83) |
| β2-microglobulin | |
| Elevated | 11 (45.83) |
| Normal | 13 (54.17) |
| ATCH | |
| Abnormal | 14 (58.33) |
| Normal | 10 (41.67) |
| LDH | |
| Elevated | 14 (58.33) |
| Normal | 10 (41.67) |
| Albumin | |
| Low | 14 (58.33) |
| Normal | 10 (41.67) |
| Ferritin | |
| Elevated | 15 (62.50) |
| Normal | 9 (37.50) |
| Blood calcium | |
| Reduced | 7 (29.17) |
| Normal | 17 (70.83) |
| Pathologic type | |
| GCB | 8 (33.33) |
| Non-GCB | 16 (66.67) |
| Ki-67 | |
| >75% | 17 (70.83) |
| ≤75% | 7 (29.17) |
| Treatment | |
| No | 6 (25.00) |
| Yes | 18 (75.00) |
| Chemotherapy time (cycles) | |
| >6 | 12 (50.00) |
| ≤6 | 12 (50.00) |
| Number of involved organs | |
| >2 | 8 (33.33) |
| ≤2 | 16 (66.67) |
| Regions of lymph node involvement | |
| >2 | 13 (54.17) |
| ≤2 | 11 (45.83) |
| SUVmax | 26.94±7.37 |
| SUVmean | 14.86±4.31 |
| MTV, cm3 | 165.71 [54.73, 347.26] |
| TLG, g | 3,324.26 [773.46, 5,658.58] |
| Maximum diameter, cm | 8.02±3.20 |
| CT attenuation value (HU) | 33.74±6.29 |
Data are presented as mean ± standard deviation/median [interquartile range]/n (%) as appropriate. ACTH: normal range is 7.2–63.3 pg/mL; β2-microglobulin: normal range is 0.8–2.4 mg/L; albumin: normal range is 35–51 g/L; ferritin: normal range is 15–200 μg/L; LDH: normal range is 100–240 IU/L. PET/CT, positron emission tomography/computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; ATCH, adrenocorticotropic hormone; LDH, lactate dehydrogenase; GCB, germinal center B-cell-like; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; HU, Hounsfield units.
Univariate analysis
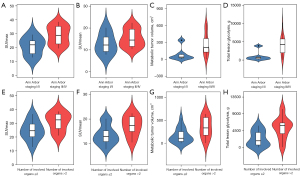
Of all the clinical indicators and baseline PET/CT parameters, Ann Arbor stage, β2-microglobulin, ATCH, number of involved organs, regions of lymph node involvement, treatment, chemotherapy cycles, SUVmax, MTV, and TLG showed association with OS (P<0.1) (Table 2, Figure 3).
Table 2
| Variables | β | HR (95% CI) | P value |
|---|---|---|---|
| Age (>60 vs. ≤60 years) | 0.46 | 1.58 (0.44–5.62) | 0.481 |
| Sex (female vs. male) | 0.74 | 2.10 (0.74–5.98) | 0.163 |
| Clinical symptoms (asymptomatic) | – | – | 0.718 |
| B symptom | −0.020 | 0.98 (0.21–4.64) | 0.979 |
| Abdominal pain | 0.432 | 1.54 (0.30–7.98) | 0.607 |
| Ann Arbor staging (III/IV vs. I/II) | 2.02 | 7.52 (0.98–57.38) | 0.021* |
| ECOG PS (2/3/4 vs. 0/1) | 0.51 | 1.66 (0.59–4.65) | 0.333 |
| IPI (2/3/4 vs. 0/1) | 0.84 | 2.32 (0.79–6.84) | 0.127 |
| β2-microglobulin (high vs. normal) | 1.22 | 3.28 (1.14–10.01) | 0.020* |
| ATCH (abnormal vs. normal) | 1.11 | 3.03 (0.96–9.59) | 0.046* |
| LDH (high vs. normal) | 0.51 | 1.67 (0.59–4.74) | 0.335 |
| Albumin (low vs. normal) | 0.67 | 1.96 (0.59–4.74) | 0.221 |
| Ferritin (high vs. normal) | 0.13 | 1.14 (0.40–3.21) | 0.808 |
| Blood calcium | 0.35 | 1.41 (0.45−4.42) | 0.554 |
| Pathologic type (GCB vs. non-GCB) | −0.36 | 0.79 (0.24–2.02) | 0.507 |
| Ki-67 (>75% vs. ≤75%) | −0.71 | 0.49 (0.17–1.41) | 0.185 |
| Treatment (no vs. yes) | −1.22 | 0.30 (0.01–0.89) | 0.020* |
| Chemotherapy cycles (>6 vs. ≤6) | −0.92 | 0.40 (0.14–1.13) | 0.072* |
| Number of involved organs (>2 vs. ≤2) | 2.02 | 7.55 (2.19–26.11) | 0.001* |
| Regions of lymph node involvement (>2 vs. ≤2) | 0.91 | 2.49 (0.85–7.30) | 0.084* |
| SUVmax (>26.7 vs. ≤26.7) | 1.14 | 3.12 (1.11–8.71) | 0.022* |
| SUVmean (>14.3 vs. ≤14.3) | 0.70 | 2.01 (0.74–5.45) | 0.170 |
| MTV (>165.7 vs. ≤165.7 cm3) | 0.89 | 2.44 (0.86–6.93) | 0.082* |
| TLG (>3,324.3 vs. ≤3,324.3 g) | 0.89 | 2.44 (0.86–6.93) | 0.082* |
| Maximum diameter (>7.9 vs. ≤7.9 cm) | 0.73 | 2.08 (0.74–5.88) | 0.168 |
| CT attenuation value (>35.2 vs. ≤35.2 HU) | 0.15 | 1.16 (0.42–3.20) | 0.776 |
*, statistically significant. PET/CT, positron emission tomography/computed tomography; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; ATCH, adrenocorticotropic hormone; LDH, lactate dehydrogenase; GCB, germinal center B-cell-like; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; HU, Hounsfield units.

Multivariate analysis
The statistically significant indicators in univariate analysis were included in the multivariate analysis. We established two Cox survival analysis models, each focusing on distinct clinical characteristics: the entire treatment process and the pre-treatment phase. The survival analysis model based on pre-treatment clinical characteristics primarily examines the impact of tumor characteristics on survival analysis, while not accounting for the influence of treatment on survival outcomes. Ann Arbor stage, β2-microglobulin, ATCH, number of involved organs, and treatment were shown to be independent prognostic factors for OS in the survival analysis model based on pre-treatment clinical characteristics (P<0.05). Ann Arbor stage, ATCH, and number of involved organs were shown to be independent prognostic factors for OS in the survival analysis model based on entire treatment process (P<0.05) (Table 3).
Table 3
| Variables | Survival analysis model based on entire treatment process | Survival analysis model based on pre-treatment process |
||||||
|---|---|---|---|---|---|---|---|---|
| β | Hazard ratio (95% CI) | P value | β | Hazard ratio (95% CI) | P value | |||
| Ann Arbor staging (III/IV vs. I/II) | 3.29 | 26.60 (2.40–294.99) | 0.008* | 2.37 | 10.94 (1.30–92.19) | 0.029* | ||
| β2-microglobulin (abnormal vs. normal) | 1.65 | 5.21 (1.47–22.69) | 0.024* | – | – | – | ||
| ATCH (abnormal vs. normal) | – | – | – | 1.56 | 4.81 (1.30–17.80) | 0.020* | ||
| Treatment (no vs. yes) | −1.58 | 0.21 (0.06–0.76) | 0.016* | – | – | – | ||
| Number of involved organs (>2 vs. ≤2) | 1.76 | 5.77 (1.47–22.69) | 0.012* | 2.33 | 10.33 (2.44–43.62) | 0.001* | ||
*, statistically significant. OS, overall survival; CI, confidence interval; ATCH, adrenocorticotropic hormone.
Correlation study analysis
Quantitative PET parameters were correlated with clinical characteristics that were statistically significant in multivariate analysis. We found that SUVmax, MTV, and TLG correlated with Ann Arbor staging (P<0.05). SUVmean and TLG correlated with the number of involved organs (P<0.05) (Table 4, Figure 4).
Table 4
| Variables | SUVmax | SUVmean | MTV | TLG |
|---|---|---|---|---|
| Ann Arbor staging | ||||
| III/IV | 28.94±6.63 | 15.48±4.08 | 208.74 (118.42, 420.99) | 4,257.75 (1,816.39, 5,303.36) |
| I/II | 20.94±6.52 | 13.01±4.81 | 41.38 (18.81, 160.39) | 608.95 (236.33, 1,610.94) |
| P value | 0.018* | 0.231 | 0.039* | 0.016* |
| β2-microglobulin | ||||
| High | 28.68±8.60 | 15.70±4.80 | 181.56 (22.50, 556.75) | 3,769.06 (454.50, 6,186.22) |
| Normal | 25.46±6.11 | 14.16±3.90 | 149.86 (63.85, 344.61) | 2,532.63 (783.52, 5,630.55) |
| P value | 0.312 | 0.395 | 0.794 | 0.794 |
| Number of involved organs | ||||
| >2 | 31.00±6.65 | 17.32±4.16 | 338.42 (159.39, 584.81) | 5,880.35 (2,923.32, 6,712.76) |
| ≤2 | 24.91±7.03 | 13.63±3.94 | 112.74 (39.26, 310.94) | 1,802.98 (531.72, 4,282.22) |
| P value | 0.054 | 0.045 | 0.050* | 0.027* |
| ATCH | ||||
| Abnormal | 28.24±8.04 | 15.44±4.41 | 278.21 (118.74, 401.15) | 4,257.75 (1,546.71, 5,969.99) |
| Normal | 25.10±6.25 | 14.06±4.24 | 90.78 (33.48, 206.49) | 1,316.72 (411.38, 3,581.25) |
| P value | 0.313 | 0.450 | 0.101 | 0.089 |
| Treatment | ||||
| No | 31.90±6.22 | 16.51±4.11 | 208.74 (109.88, 329.71) | 4,257.75 (2,184.40, 5,393.16) |
| Yes | 25.28±7.11 | 14.31±4.34 | 137.30 (43.49, 420.99) | 2,181.22 (686.17, 5,717.23) |
| P value | 0.055 | 0.289 | 1.000 | 0.505 |
Data are presented as mean ± standard deviation/median (interquartile range) as appropriate. *, statistically significant. PET/CT, positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; ATCH, adrenocorticotropic hormone.
Discussion
Identification of the poor prognostic group could be helpful for guiding further treatment strategies in PA-DLBCL patients. Results from our present preliminary study indicate that Ann Arbor stage, β2-microglobulin, ATCH, the number of involved organs, regions of lymph node involvement, treatment, chemotherapy cycles, SUVmax, MTV, and TLG are associated with OS. In addition, Ann Arbor stage, β2-microglobulin, ATCH, number of involved organs, and treatment were shown to be independent prognostic factors for OS in the survival analysis model based on pre-treatment clinical characteristics. Notably no quantitative PET parameters were available in the multivariate analysis as an independent risk factor for OS, but in the correlation analysis, we found that SUVmax, MTV, and TLG correlated with Ann Arbor staging, and that SUVmean and TLG correlated with the number of involved organs.
Numerous clinical studies have reported prognostic factors associated with DLBCL, and it is crucial to identify high-risk groups before initiating treatment for selecting appropriate clinical strategies. Compared to other DLBCL subtypes, PA-DLBCL carries a worse prognosis. Li et al. (36) reported 5- and 10-year OS rates of 19.17% and 3.33%, respectively, for patients with adrenal DLBCL. In this study, the median survival of patients with PA-DLBCL was 17.5 months, which suggests a possible correlation with the rapid disease progression. Compared to patients who received no treatment, we found that undergoing chemotherapy was a positive prognostic factor for predicting OS. Previous studies have reported that the most commonly used chemotherapy regimens were CHOP or R-CHOP (37-40). Zhang et al. conducted a series tracking the outcomes of 14 patients with PA-DLBCL and endorsed the use of the R-CHOP regimen, reporting that achieving complete remission (CR) following R-CHOP was predictive of improved survival (41). Similarly, Kim et al. suggested that R-CHOP combination chemotherapy was an effective first-line regimen for PA-DLBCL (9). The findings of Lu et al. (42) suggest that specific extranodal involved sites have significant prognostic value in DLBCL patients treated with R-CHOP. Hui et al. (43) also identified the number of extranodal involvement sites as an important prognostic factor in DLBCL patients treated with R-CHOP. Similarly, our study found that the number of extra nodal lesions other than adrenal is associated with poorer OS rates. Secondary CNS involvement in DLBCL, including relapse or progression, significantly impacts treatment efficacy (44-46). Some researchers have reported prophylactic treatments to reduce the recurrence of CNS relapse, including intrathecal (IT) injection chemotherapy alone and high-dose methotrexate-based regimens and/or cytarabine (47). However, the lack of effective biological therapies due to the impermeability of the blood-brain barrier results in an unclear optimal prophylactic strategy (48). Our study only included four patients with CNS involvement, with worse prognosis than those without CNS involvement, with only 1 case currently alive.
Existing prognostic scoring systems such as the IPI can assess the prognosis of most patients with DLBCL, but some patients with similar IPI scores still have different long-term survival rates (49). Quantitative parameters derived from 18F-FDG PET have demonstrated predictive value for assessing outcomes in patients with DLBCL (27-29,49-51). The standardized uptake value (SUV) is a widely utilized semiquantitative index of 18F-FDG metabolic rate, known for its ease of calculation and noninvasive nature (52). Chihara et al. (51) assessed the prognostic significance of baseline SUVmax in 169 DLBCL patients undergoing R-CHOP treatment. Multivariate analysis revealed that high SUVmax is a significant adverse prognostic factor for both progression-free survival (PFS) and OS. Positive findings on interim PET performed between 2 and 4 cycles of chemotherapy indicate a higher likelihood of disease relapse in patients with DLBCL. Fuertes et al. (53) conducted an assessment to determine whether interim PET could serve as a prognostic indicator for OS and PFS in DLBCL patients. Their findings revealed that ΔSUVmax cut-off value of 76% [95% confidence interval (CI): 62.7–89.2%] and 75% (95% CI: 54.6–95.4%) was optimal for predicting differences in PFS and OS, respectively. Lin et al. (52) reported that assessing therapeutic response using SUV during initial chemotherapy enhances the prognostic value of early 18F-FDG PET in patients with DLBCL. Our results also demonstrate the prognostic value of SUVmax, indicating that patients with PA-DLBCL have a more favorable prognosis when SUVmax is ≤26.7 compared to >26.7, and correlating with Ann Arbor staging. However, it is susceptible to measurement variability caused by factors such as imaging delay after 18F-FDG injection, partial volume effects, and the applied normalization scheme (54-56).
Following the development of software programs, the MTV and TLG on PET/CT serve as measures of tumor metabolic activity, offering valuable information for assessing efficacy and prognosis in patients with DLBCL (57,58). In a meta-analysis conducted by Xie et al. (59), involving 703 patients from seven retrospective trials, it was observed that high MTV is significantly associated with decreased survival among DLBCL patients undergoing R-CHOP treatment. Malek et al. (60) conducted a comparison between gradient- or threshold-based methods for measuring MTV and SUVmax on interim PET analyses to assess their predictive ability for PFS in patients with DLBCL following initial therapy. The results demonstrated that patients who achieved a ΔSUVmax of 472% on interim PET, and those with a ΔMTV of 452% exhibited a significantly improved PFS (P=0.02). Similarly, Shagera et al. (1) and Song et al. (23) identified pretreatment MTV as an independent predictor of survival in DLBCL patients. All of these findings suggest that MTV and TLG hold significant potential for predicting the prognosis of patients with DLBCL. However, it is worth noting that the measurement methods for MTV and TLG are relatively complex, and a consensus on the most accurate segmentation method has not been reached. Standardization and operational specifications need to be established (61,62). In previous literature, Ann Arbor staging and the number of involved organs have been reported as poor prognostic factors for DLBCL. In our study, we observed correlations between MTV and TLG with Ann Arbor staging and number of involved organs (42,63-65). However, in the multifactorial analysis, MTV and TLG were not identified as independent predictors. This observation may be attributed to the presence of large adrenal lesions with internal necrosis in the majority of patients in our study group. The necrotic condition within the tumor significantly affects the magnitude of MTV and TLG measurements. The absence of FDG uptake in the internal necrotic area results in lower MTV and TLG values, leading to an underestimation of the true tumor burden and a reduction in their predictive efficacy. Subsequent studies with increased sample sizes or prolonged follow-up periods may reveal the predictive value of MTV and TLG. We are actively working on this task. Given the potential prognostic value of these FDG-PET parameters, it is essential to carefully consider treatment approaches for patients with higher values. In clinical practice, a more aggressive treatment strategy may indeed be considered for patients with poor prognostic FDG-PET parameters to improve their outcomes (7). Aggressive treatment options may include intensified chemotherapy regimens, targeted therapies, radiation therapy, or stem cell transplantation, depending on individual patient characteristics and disease stage. However, it is crucial to emphasize that treatment decisions should be made on a case-by-case basis, taking into account the overall health status of the patient, disease extent, presence of comorbidities, and treatment-related toxicities. Additionally, further research and prospective studies are needed to validate the predictive value of FDG-PET parameters. In recent years, there has been growing interest in the role of radiomics, particularly in the context of artificial intelligence, as a potential tool for predicting treatment outcomes in various malignancies, including DLBCL. Eertink et al. (28) demonstrated the value of 18F-FDG PET baseline radiomics features in improving the prediction of treatment outcomes in DLBCL. The authors reported promising results, suggesting that radiomics analysis may offer valuable prognostic insights. Therefore, we encourage further investigation into the role of radiomics in the prognosis of this specific lymphoma subtype.
The present study has certain limitations, and no quantitative FDG-PET parameters indicated predictive factors in our multivariate analysis. We recognize the potential biases that could be addressed by a prospective study. Firstly, patients from two centers were included in our study, the low prevalence of PA-DLBCL and limited data posed challenges in conducting further investigations. To reduce this limitation, future studies will focus on expanding the sample size, potentially through multi-center collaborations, to enhance the statistical power and generalizability of our findings. Secondly, variations in PET/CT imaging equipment between two centers could have introduced variability in the imaging results. Standardization of imaging protocols is crucial to ensure consistent and reliable measurements across different centers. In a prospective study, it would be feasible to implement standardized imaging protocols from the outset, leading to more robust and comparable data. Thirdly, our study had 6 patients who did not receive treatment, and this could have influenced the prognosis analysis. To address this issue, we established two Cox survival analysis models, each focusing on distinct clinical characteristics: the entire treatment process and the pre-treatment phase. Additionally, the follow-up time for some cases in this study was relatively short, with only 15 patients completing more than 2 years of follow-up. Meanwhile, we have plans to conduct further analysis on the 3- and 5-year PFS, which would allow for longer and more comprehensive follow-up periods, providing a clearer understanding of the long-term outcomes of PA-DLBCL patients. Lastly, advancements in PET/CT technology and the emergence of artificial intelligence could influence the prognostic value of PET/CT in PA-DLBCL in the future. Prospective studies could incorporate these technological advancements, enabling more refined and accurate prognostic predictive information for the evaluation of PA-DLBCL during the chemotherapy.
Conclusions
PA-DLBCL is characterized by a low incidence and a poor prognosis, encompassing cases with isolated involvement of adrenal tissue as well as cases with additional extra-adrenal organ manifestations. Prognostic factors for OS include Ann Arbor stage, β2-microglobulin, ATCH, number of involved organs, and treatment. Baseline 18F-FDG PET/CT quantization parameters showed correlations with Ann Arbor staging and number of involved organs. Increasing the sample size or prolonging the follow-up period may reveal the predictive value of PET/CT quantization parameters. There is a need to enhance FDG-PET examinations for improved prognostic evaluation of tumors, which would be valuable for standardizing treatment.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-803/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-803/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Peking University First Hospital and the First Affiliated Hospital of Zhengzhou University, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shagera QA, Cheon GJ, Koh Y, Yoo MY, Kang KW, Lee DS, Kim EE, Yoon SS, Chung JK. Prognostic value of metabolic tumour volume on baseline (18)F-FDG PET/CT in addition to NCCN-IPI in patients with diffuse large B-cell lymphoma: further stratification of the group with a high-risk NCCN-IPI. Eur J Nucl Med Mol Imaging 2019;46:1417-27. [Crossref] [PubMed]
- Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. N Engl J Med 2021;384:842-58. [Crossref] [PubMed]
- Kawano T, Tsuyuki Y, Suzuki Y, Shimada K, Kato S, Takahara T, Mori M, Nakaguro M, Sakakibara A, Nakamura S, Satou A. Clinicopathologic Analysis of Primary Adrenal Diffuse Large B-Cell Lymphoma: A Reappraisal of 23 Japanese Patients Based on EBV Association and PD-L1 Expression in Tumor Cells. Am J Surg Pathol 2021;45:1606-15. [Crossref] [PubMed]
- Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol 2018;19:38. [Crossref] [PubMed]
- Yu K, Xue Q, Zhou F, Tian H, Xiang Q, Chen T, Ren Y. A Novel Diagnostic Model for Primary Adrenal Lymphoma. Front Endocrinol (Lausanne) 2021;12:636658. [Crossref] [PubMed]
- Mozos A, Ye H, Chuang WY, Chu JS, Huang WT, Chen HK, Hsu YH, Bacon CM, Du MQ, Campo E, Chuang SS. Most primary adrenal lymphomas are diffuse large B-cell lymphomas with non-germinal center B-cell phenotype, BCL6 gene rearrangement and poor prognosis. Mod Pathol 2009;22:1210-7. [Crossref] [PubMed]
- Rashidi A, Fisher SI. Primary adrenal lymphoma: a systematic review. Ann Hematol 2013;92:1583-93. [Crossref] [PubMed]
- Lee KR, Koh J, Jeon YK, Kwon HJ, Lee JO, Paik JH. Clinicopathologic implication of PD-L1 gene alteration in primary adrenal diffuse large B cell lymphoma. J Pathol Transl Med 2022;56:32-9. [Crossref] [PubMed]
- Kim YR, Kim JS, Min YH, Hyunyoon D, Shin HJ, Mun YC, et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the Consortium for Improving Survival of Lymphoma (CISL). J Hematol Oncol 2012;5:49. [Crossref] [PubMed]
- Majidi F, Martino S, Kondakci M, Antke C, Haase M, Chortis V, et al. Clinical spectrum of primary adrenal lymphoma: results of a multicenter cohort study. Eur J Endocrinol 2020;183:453-62. [Crossref] [PubMed]
- Wight JC, Chong G, Grigg AP, Hawkes EA. Prognostication of diffuse large B-cell lymphoma in the molecular era: moving beyond the IPI. Blood Rev 2018;32:400-15. [Crossref] [PubMed]
- Gleeson M, Counsell N, Cunningham D, Lawrie A, Clifton-Hadley L, Hawkes E, McMillan A, Ardeshna KM, Burton C, Chadwick N, Gambell J, Smith P, Mouncey P, Pocock C, Radford J, Davies J, Turner D, Kruger A, Johnson P, Linch D. Prognostic indices in diffuse large B-cell lymphoma in the rituximab era: an analysis of the UK National Cancer Research Institute R-CHOP 14 versus 21 phase 3 trial. Br J Haematol 2021;192:1015-9. [Crossref] [PubMed]
- Ruppert AS, Dixon JG, Salles G, Wall A, Cunningham D, Poeschel V, Haioun C, Tilly H, Ghesquieres H, Ziepert M, Flament J, Flowers C, Shi Q, Schmitz N. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 2020;135:2041-8. [Crossref] [PubMed]
- Pellegrino F, Scabbia F, Merlo A, Perrucci L, Aliberti L, Urso A, Ambrosio MR, Cuneo A, Galeotti R, Giganti M. Spontaneously reversible adrenal nodules in primary diffuse large B-cell testicular lymphoma mimicking an extranodal involvement: A case report. Radiol Case Rep 2021;16:2168-73. [Crossref] [PubMed]
- Kobe C, Dietlein M, Hellwig D. PET/CT for Lymphoma Post-therapy Response Assessment in Hodgkin Lymphoma and Diffuse Large B-cell Lymphoma. Semin Nucl Med 2018;48:28-36. [Crossref] [PubMed]
- Juweid ME, Wiseman GA, Vose JM, Ritchie JM, Menda Y, Wooldridge JE, Mottaghy FM, Rohren EM, Blumstein NM, Stolpen A, Link BK, Reske SN, Graham MM, Cheson BD. Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol 2005;23:4652-61. [Crossref] [PubMed]
- Kim J, Song YS, Lee JS, Lee WW, Kim SE. Risk stratification of diffuse large B-cell lymphoma with interim PET-CT based on different cutoff Deauville scores. Leuk Lymphoma 2018;59:340-7. [Crossref] [PubMed]
- Cottereau AS, Meignan M, Nioche C, Capobianco N, Clerc J, Chartier L, Vercellino L, Casasnovas O, Thieblemont C, Buvat I. Risk stratification in diffuse large B-cell lymphoma using lesion dissemination and metabolic tumor burden calculated from baseline PET/CT Ann Oncol 2021;32:404-11. [Crossref] [PubMed]
- Michallet AS, Trotman J, Tychyj-Pinel C. Role of early PET in the management of diffuse large B-cell lymphoma. Curr Opin Oncol 2010;22:414-8. [Crossref] [PubMed]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571-8. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Györke T, Carr R, Cerci JJ, Meneghetti C, Redondo F, Celli M, Gorospe C, Auewarakul CU, Jorgov L, Paez D, Fanti S. Combined Visual and Semiquantitative Evaluation Improves Outcome Prediction by Early Midtreatment (18)F-FDG PET in Diffuse Large B-Cell Lymphoma. J Nucl Med 2020;61:999-1005. [Crossref] [PubMed]
- Song MK, Yang DH, Lee GW, Lim SN, Shin S, Pak KJ, Kwon SY, Shim HK, Choi BH, Kim IS, Shin DH, Kim SG, Oh SY. High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large B cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res 2016;42:1-6. [Crossref] [PubMed]
- Marchetti L, Perrucci L, Pellegrino F, Baroni L, Merlo A, Tilli M, Rambaldi I, Maietti E, Carnevale A, Bartolomei M, Giganti M. Diagnostic Contribution of Contrast-Enhanced CT as Compared with Unenhanced Low-Dose CT in PET/CT Staging and Treatment Response Assessment of (18)F-FDG-Avid Lymphomas: A Prospective Study. J Nucl Med 2021;62:1372-9. [Crossref] [PubMed]
- Meignan M, Hutchings M, Schwartz LH. Imaging in Lymphoma: The Key Role of Fluorodeoxyglucose-Positron Emission Tomography. Oncologist 2015;20:890-5. [Crossref] [PubMed]
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014;32:3048-58. [Crossref] [PubMed]
- A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987-94. [Crossref] [PubMed]
- Eertink JJ, van de Brug T, Wiegers SE, Zwezerijnen GJC, Pfaehler EAG, Lugtenburg PJ, van der Holt B, de Vet HCW, Hoekstra OS, Boellaard R, Zijlstra JM. (18)F-FDG PET baseline radiomics features improve the prediction of treatment outcome in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 2022;49:932-42. [Crossref] [PubMed]
- Campiotti L. DE Palma D, Guasti L, Proserpio I, Casagrande S, Schiorlin I, Bolzacchini E, Suter M, Ogliari F, Squizzato A. Baseline PET as prognostic index in diffuse large B-cell lymphoma and grade IIIb follicular lymphoma: a retrospective study of a single-center experience. Q J Nucl Med Mol Imaging 2021;65:59-63. [Crossref] [PubMed]
- Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin's lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol 2003;14:131-9. [Crossref] [PubMed]
- Laurent C, Casasnovas O, Martin L, Chauchet A, Ghesquieres H, Aussedat G, Fornecker LM, Bologna S, Borot S, Laurent K, Bouillet B, Verges B, Petit JM. Adrenal lymphoma: presentation, management and prognosis. QJM 2017;110:103-9. [PubMed]
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989;7:1630-6. [Crossref] [PubMed]
- Chen Z, Zou Y, Liu W, Guan P, Tao Q, Xiang C, Zhang W, Ye Y, Yan J, Zhao S. Morphologic Patterns and the Correlation With MYD88 L265P, CD79B Mutations in Primary Adrenal Diffuse Large B-Cell Lymphoma. Am J Surg Pathol 2020;44:444-55. [Crossref] [PubMed]
- Meignan M, Sasanelli M, Casasnovas RO, Luminari S, Fioroni F, Coriani C, Masset H, Itti E, Gobbi PG, Merli F, Versari A. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging 2014;41:1113-22. [Crossref] [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]
- Li S, Wang Z, Wu Z, Zhuang H, Xu Y. Clinical characteristics and outcomes of primary adrenal diffuse large B cell lymphoma in a large contemporary cohort: a SEER-based analysis. Ann Hematol 2019;98:2111-9. [Crossref] [PubMed]
- Melchardt T, Egle A, Greil R. How I treat diffuse large B-cell lymphoma. ESMO Open 2023;8:100750. [Crossref] [PubMed]
- Bauduer F, Delmer A, Le Tourneau A, Guettier G, Alexandre JH, Zittoun R, Bernadou A. Primary adrenal lymphoma. Acta Haematol 1992;88:213-5. [Crossref] [PubMed]
- Sasagawa I, Sadamori N, Itoyama T, Tsukasaki K, Nakamura H, Tomonaga M, Kishikawa M. Primary adrenal lymphoma with chromosomal abnormalities. Acta Haematol 1995;94:156-62. [Crossref] [PubMed]
- Ichikawa S, Fukuhara N, Inoue A, Katsushima H, Ohba R, Katsuoka Y, Onishi Y, Yamamoto J, Sasaki O, Nomura J, Fukuhara O, Ishizawa K, Ichinohasama R, Harigae H. Clinicopathological analysis of primary adrenal diffuse large B-cell lymphoma: effectiveness of rituximab-containing chemotherapy including central nervous system prophylaxis. Exp Hematol Oncol 2013;2:19. [Crossref] [PubMed]
- Zhang J, Sun J, Feng J, Luo Y, Ling Q, Wu S, Zeng X, Liang Z. Primary adrenal diffuse large B cell lymphoma: a clinicopathological and molecular study from China. Virchows Arch 2018;473:95-103. [Crossref] [PubMed]
- Lu CS, Chen JH, Huang TC, Wu YY, Chang PY, Dai MS, Chen YC, Ho CL. Diffuse large B-cell lymphoma: sites of extranodal involvement are a stronger prognostic indicator than number of extranodal sites in the rituximab era. Leuk Lymphoma 2015;56:2047-55. [Crossref] [PubMed]
- Hui D, Proctor B, Donaldson J, Shenkier T, Hoskins P, Klasa R, Savage K, Chhanabhai M, Gascoyne RD, Connors JM, Sehn LH. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma 2010;51:1658-67. [Crossref] [PubMed]
- Ma J, Li Q, Shao J, Ma Y, Lin Z, Kang H, Chen B. Central Nervous System Involvement in Patients with Diffuse Large B Cell Lymphoma: Analysis of the Risk Factors and Prognosis from a Single-Center Retrospective Cohort Study. Cancer Manag Res 2019;11:10175-85. [Crossref] [PubMed]
- Abramson JS. High-dose chemotherapy and autologous stem cell transplantation for secondary central nervous system lymphoma: many are called, but few are chosen. Haematologica 2013;98:662-4. [Crossref] [PubMed]
- Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: a systematic review and meta-analysis. Leuk Lymphoma 2014;55:509-14. [Crossref] [PubMed]
- Cheah CY, Herbert KE, O'Rourke K, Kennedy GA, George A, Fedele PL, Gilbertson M, Tan SY, Ritchie DS, Opat SS, Prince HM, Dickinson M, Burbury K, Wolf M, Januszewicz EH, Tam CS, Westerman DA, Carney DA, Harrison SJ, Seymour JF. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 2014;111:1072-9. [Crossref] [PubMed]
- Zhang Y, Li Y, Zhuang Z, Wang W, Wei C, Zhao D, Zhou D, Zhang W. Preliminary Evaluation of Zanubrutinib-Containing Regimens in DLBCL and the Cerebrospinal Fluid Distribution of Zanubrutinib: A 13-Case Series. Front Oncol 2021;11:760405. [Crossref] [PubMed]
- Zhu L, Meng Y, Guo L, Zhao H, Shi Y, Li S, Wang A, Zhang X, Shi J, Zhu J, Xu K. Predictive value of baseline (18)F-FDG PET/CT and interim treatment response for the prognosis of patients with diffuse large B-cell lymphoma receiving R-CHOP chemotherapy. Oncol Lett 2021;21:132. [Crossref] [PubMed]
- Wang M, Xu H, Xiao L, Song W, Zhu S, Ma X. Prognostic Value of Functional Parameters of (18)F-FDG-PET Images in Patients with Primary Renal/Adrenal Lymphoma. Contrast Media Mol Imaging 2019;2019:2641627. [Crossref] [PubMed]
- Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, Morishima Y. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol 2011;93:502-8. [Crossref] [PubMed]
- Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, Paone G, Talbot JN, Rahmouni A, Meignan M. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med 2007;48:1626-32. [Crossref] [PubMed]
- Fuertes S, Setoain X, Lopez-Guillermo A, Carrasco JL, Rodríguez S, Rovira J, Pons F. Interim FDG PET/CT as a prognostic factor in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 2013;40:496-504. [Crossref] [PubMed]
- Keyes JW Jr. SUV: standard uptake or silly useless value? J Nucl Med 1995;36:1836-9. [PubMed]
- Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med 2004;45:1431-4. [PubMed]
- Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004;45:1519-27. [PubMed]
- Frood R, Burton C, Tsoumpas C, Frangi AF, Gleeson F, Patel C, Scarsbrook A. Baseline PET/CT imaging parameters for prediction of treatment outcome in Hodgkin and diffuse large B cell lymphoma: a systematic review. Eur J Nucl Med Mol Imaging 2021;48:3198-220. [Crossref] [PubMed]
- Feng X, Wen X, Li L, Sun Z, Li X, Zhang L, Wu J, Fu X, Wang X, Yu H, Ma X, Zhang X, Xie X, Han X, Zhang M. Baseline Total Metabolic Tumor Volume and Total Lesion Glycolysis Measured on 18F-FDG PET-CT Predict Outcomes in T-Cell Lymphoblastic Lymphoma. Cancer Res Treat 2021;53:837-46. [Crossref] [PubMed]
- Xie M, Wu K, Liu Y, Jiang Q, Xie Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol 2015;32:446. [Crossref] [PubMed]
- Malek E, Sendilnathan A, Yellu M, Petersen A, Fernandez-Ulloa M, Driscoll JJ. Metabolic tumor volume on interim PET is a better predictor of outcome in diffuse large B-cell lymphoma than semiquantitative methods. Blood Cancer J 2015;5:e326. [Crossref] [PubMed]
- Barrington SF, Meignan M. Time to Prepare for Risk Adaptation in Lymphoma by Standardizing Measurement of Metabolic Tumor Burden. J Nucl Med 2019;60:1096-102. [Crossref] [PubMed]
- Gallamini A. In Search of Platinum Meter Bar for Measurement of Metabolic Tumor Volume in Lymphoma. J Nucl Med 2019;60:1094-5. [Crossref] [PubMed]
- Yuan T, Chen X, Zhang Y, Wei M, Zhu H, Yang Z, Wang X. A novel prognostic index for diffuse large B-cell lymphoma combined baseline metabolic tumour volume with clinical and pathological risk factors. Nucl Med Commun 2023;44:622-30. [Crossref] [PubMed]
- Yang S, Chang W, Zhang B, Shang P. What factors are associated with the prognosis of primary testicular diffuse large B-cell lymphoma? A study based on the SEER database. J Cancer Res Clin Oncol 2023;149:10269-78. [Crossref] [PubMed]
- Varelas AN, Eggerstedt M, Ganti A, Tajudeen BA. Epidemiologic, prognostic, and treatment factors in sinonasal diffuse large B -cell lymphoma. Laryngoscope 2019;129:1259-64. [Crossref] [PubMed]


