
Application of simple ultrasound Doppler hemodynamic parameters in the diagnosis of severe renal artery stenosis in routine clinical practice
Introduction
Renovascular hypertension is the most common type of secondary hypertension, affecting approximately 5–10% of the general population, with an even higher prevalence among patients with severe hypertension or end-stage renal disease and among older adults (1,2). Renal artery stenosis (RAS) is the most common cause of renovascular hypertension and is mainly secondary to atherosclerotic disease and fibromuscular dysplasia (FMD) (3). Medical therapy remains the cornerstone of treatment for RAS, and renal revascularization should be considered in patients with anatomically and functionally severe RAS (2-7). Although optimal therapy in patients with RAS is controversial and should be determined based on the particular etiology or clinical scenario, accurate diagnosis and evaluation are reasonable and necessary.
Many imaging modalities are available for the diagnosis of RAS. Contrast-enhanced computed tomography angiography (CTA), magnetic resonance angiography (MRA), and Doppler ultrasound (DUS) are recommended for the establishment of an RAS diagnosis, and digital subtraction angiography (DSA) is considered the gold standard and predominantly used for RAS confirmation and intervention (1). Although CTA and MRA provide accurate anatomical images of renal arteries, there are still some limitations in their clinical application related to radiation absorption, specific metallic or electrical implants, devices, foreign bodies, imposition of severe claustrophobia, and impaired renal function (1,3,8-14).
DUS is an attractive technique owing to its noninvasiveness, simplicity, and reproducibility and is associated with numerous parameters and abnormal criteria capable of indicating possible renovascular disease (1). DUS is recommended in first-line imaging for the diagnosis of RAS in the European Society of Cardiology/European Society for Vascular Surgery (ESC/ESVS) practice guidelines (2). In the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) practice guidelines, DUS is recommended as a screening test to establish the diagnosis of RAS (15). Over the past few years, the rapid development of new technologies, such as contrast-enhanced ultrasound (CEUS), has further increased the accuracy of RAS diagnosis (16). CEUS can clearly show the renal arteries and is remarkably consistent with DSA, the widely acknowledged gold standard, in the diagnosis of RAS ≥70% (17). DUS and CEUS can accurately identify patients with severe RAS who are suitable candidates for angiography, thus effectively avoiding unnecessary revascularization, the risk of complications, and health care costs.
New ultrasound technology, such as CEUS, provides added value in the diagnosis of RAS. However, due to a few factors, including the higher cost of contrast agents and the specialized training required for performing and interpreting CEUS examinations, the popularity of CEUS in renal artery examination is not particularly high, especially in basic-level hospitals in China. Conventional DUS, which can reflect the degree of stenosis and intrarenal hemodynamic changes with morphologic recognition and quantitative analysis of the Doppler waveform, remains the leading method to diagnose RAS. However, some factors limit the clinical application and popularity of DUS. For one, renal artery examination is relatively complicated and time-consuming requiring examiners with systematic training and prior experience in abdominal and vascular DUS examination, which greatly reduces the detection rate of RAS. For another, over the past few decades, many parameters and thresholds for the diagnosis of RAS have been proposed. Peak systolic velocity (PSV) in the main renal artery, the ratio of the peak velocities in the renal artery and the aorta (RAR), the ratio of the peak velocities in the renal artery and the segmental artery (RSR), and the ratio of the peak velocities in the renal artery and the interlobar artery (RIR) are all useful for the diagnosis of RAS (18-27). However, the correct selection of these direct or indirect parameters and their optimal thresholds remain controversial. Overall, further research and standardization efforts are necessary to overcome these limitations and optimize the clinical application of DUS for the evaluation of RAS.
PSV is the most widely used and accurately measured Doppler parameter and can directly reflect the hemodynamic changes in stenosis (1,3). Higher velocity may correlate with a greater pressure differential across the stenosis (3). In this study, we retrospectively analyzed PSV and PSV-related DUS parameters and explored a simple, accurate, fast, and efficient (SAFE) DUS examination method for screening and diagnosing severe RAS in routine clinical practice. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-605/rc).
Methods
Patient selection
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), approved by the Institutional Review Board of Beijing Hospital (No. 2018BJYYEC-043-02), and registered in the China Clinical Trial Registration Center (No. ChiCTR1800016252). All patients or their legal guardians signed an informed consent form before examination. From January 2018 to September 2021, a total of 1,110 patients underwent renal artery CEUS examinations in our institute and were retrospectively analyzed. All patients were consecutively enrolled and were first examined by renal artery CEUS followed by DSA or CTA to determine the presence and degree of RAS.
The inclusion criteria were the following: (I) RAS first diagnosed with ultrasound (30%≤ stenosis rate ≤99%); (II) complete DUS data, including PSV in the aorta, main renal artery, segmental artery, and interlobar artery; and (III) RAS (stenosis rate ≥30%) confirmed via DSA or CTA examination.
Meanwhile, the exclusion criteria were the following: (I) normal renal artery or RAS rate <30%; (II) renal artery occlusion; (III) diffuse severe stenosis of the main renal artery or high-speed blood flow at the stenosis that could not be measured; (IV) abdominal aorta stenosis or abdominal aortic aneurysm; (V) patients with severe cardiac or pulmonary insufficiency or who were allergic to the ultrasound contrast agent; (VI) pregnant and lactating patients; and (VII) poor quality ultrasound images.
Study protocol and imaging analysis
Renal artery CEUS examinations were performed as follows. First, the patient’s detailed medical history was obtained to determine the purpose of the examination. Second, basic DUS parameters, such as PSV, were acquired. Third, a CEUS examination of the renal artery was performed.
All ultrasound images were interpreted by two experienced ultrasound doctors (over 5 years of experience in vascular and abdominal ultrasound) working in consensus and blinded to other diagnostic results.
Ultrasound instruments and contrast agent
The following three types of ultrasound instruments and contrast conditions were used in this study: (I) RS80A (Samsung, Seoul, South Korea); (II) Aplio i800 (Canon, Otahara City, Japan); and (III) LOGIQ E8 (GE HealthCare, Chicago, USA). The CEUS contrast agent was SonoVue (Bracco Spa, Milan, Italy).
Renal artery DUS
The patients fasted for more than 8 hours to reduce intestinal movement and gas disturbances. We first measured the basic Doppler parameters. The contents of the DUS parameter measurements included the peak systolic flow velocity of the abdominal aorta, PSV at the stenosis of the main renal artery, PSV of the renal segmental artery, and PSV of the renal interlobar artery (Figure 1).
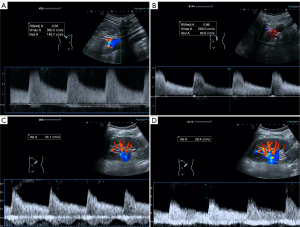
Details of specific measurement method were as follows: (I) the abdominal aortic blood flow velocity was measured at 1–1.5 cm below the superior mesenteric artery. (II) The PSV at the stenosis of the main renal artery was measured on the modified lateral lumbar coronal section. The patient’s back was placed at a 60º–90º angle with the operating bed when he or she was in the right lateral decubitus position and a 45º–60º angle when he or she was in the left lateral decubitus position. The purpose of this placement was to relax the abdominal wall into a soft state. Then, the direction of the probe was adjusted to first display the long axis of the abdominal aorta, and the probe was pressed deeply to clearly display the opening of the renal artery. (III) The segment arteries and interlobar arteries in the middle of the kidney with the smallest angle to the direction of the sound beam were selected for measurement.
Renal artery CEUS
In this study, it was necessary to display the entire main renal artery as much as possible and then fix the probe to enter the angiography mode.
Following the bolus injection of 1 mL of SonoVue into the median cubital vein, 5 mL of 0.9% sodium chloride was quickly injected to flush the cannula. The recording was started at the same time as contrast agent injection, and 30 seconds of dynamic imaging was recorded. The clearest CEUS image was selected to measure the renal artery lumen diameter and stenosis rate. All CEUS examination results of 106 renal arteries were then compared with those of DSA or CTA examination to confirm the degree of stenosis. In cases where CEUS examination revealed a RAS greater than 70%, DSA examination was performed for confirmation. For cases where CEUS examination indicated a RAS less than 70%, DSA or CTA examination was performed for confirmation.
Statistical methods
The data were analyzed using SPSS version 24.0 (IBM Corp., NY, USA) and MedCalc version 20.111 (MedCalc Software, Ostend, Belgium). Continuous variables are expressed as the median and interquartile range (IQR), while categorical variables are expressed as the number of cases and percentages. Mann-Whitney test was used for comparison between the continuous variables. In order to identify potential candidates for revascularization more effectively, all enrolled patients were divided into two groups based on the degree of diameter reduction: a severe stenosis group (diameter reduction ≥70%) and a non-severe stenosis group (diameter reduction <70%). Logistic regression analysis was performed to determine the independent predictors for severe stenosis among the DUS parameters, including PSV in the main renal artery, RAR, RSR, and RIR. The diagnostic performance of different Doppler parameters was evaluated using the receiver operating characteristic (ROC) curve and area under the curve (AUC). The optimal thresholds were determined using the Youden index derived from the ROC curve to balance sensitivity and specificity. The threshold value that corresponded to the highest Youden index was chosen as the cutoff point. Various performance measures, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and total accuracy of Doppler hemodynamic parameters were calculated and displayed. MedCalc was used to generate ROC curves for different Doppler variables and compare their diagnostic performance based on the AUC values. First, the differences in AUC values among PSV, RAR, RSR, and RIR were compared. Subsequently, the AUC of the combined 4 metrics was compared with the individual AUC values. A P value <0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the patient population
A total of 85 patients, comprising 106 stenosed renal arteries, were enrolled in this study. Their screening and analysis flow diagram is presented in Figure S1, while the baseline characteristics of the patients are detailed in Table 1. The median age of the patients was 71 (range, 64–77) years, and 4 were younger than 18 years old. RAS was mainly secondary to atherosclerosis disease (81 in 85 patients) in this group. A total of 101 lesions were located in the proximal segment or ostium of the main renal arteries, 4 in the middle, and 1 in the distal segment. Based on DSA findings, 62 renal arteries with severe stenosis (diameter reduction ≥70%) were identified. Compared with other angiographic techniques, CEUS overestimated 2 cases and underestimated 4 cases in identifying severe RAS. No adverse events occurred in the study.
Table 1
| Variable | Patients with RAS (N=85) | Stenosed renal arteries (N=106) |
|---|---|---|
| Age (years) | 71 [64–77] | – |
| Male sex | 58 [68] | – |
| Bilateral RAS | 21 [25] | – |
| RAS secondary to atherosclerosis disease | 81 [95] | – |
| Left main renal artery | – | 51 [48] |
| RAS located in the proximal segment of the main renal artery | – | 101 [95] |
| Severe RAS (diameter reduction ≥70%) | – | 62 [58] |
Data are shown as median [IQR] or n [%]. RAS, renal artery stenosis; IQR, interquartile range.
Basic Doppler parameters for the renal artery
The basic Doppler parameter measurements included PSV of the abdominal aorta, PSV at the stenosis of the main renal artery, PSV of the renal segmental artery, and PSV of the renal interlobar artery. For the severe stenosis group, the median PSVs of stenosis, segmental artery, and interlobar artery were 301.1, 33.5, and 25.0 cm/s, respectively. The median RAR, RSR, and RIR were 4.2, 9.2, and 12.0, respectively. Other median values and IQRs of basic DUS parameters recorded from renal arteries are presented in the Table S1. The ultrasound contrast agent was injected after the acquisition of basic DUS parameters. CEUS was used to locate and confirm the narrowest point of the main renal artery. The clearest CEUS image was selected to measure the RAS rate and lumen diameter.
Establishment of the optimal thresholds for the diagnosis of severe RAS
Doppler data were reviewed to identify the parameters that best predicted angiographic RAS ≥70%. Logistic regression analysis was performed based on the stenosis degree, and patients were categorized into two groups (diameter reduction ≥70% and diameter reduction <70%). Significant differences in PSV, RAR, RSR, and RIR were noted between the two groups (P<0.05) and are detailed in Table S1. An ROC curve was constructed to demonstrate the performance of these parameters in the detection of severe RAS. The PSV of the stenosed main renal artery (PSVmain), RAR, RSR, and RIR exhibited good diagnostic efficiency (AUC >0.8). The optimal thresholds for the diagnosis of severe RAS obtained via ROC curves were 249.5 cm/s, 2.94, 5.1, and 7.5 for PSV, RAR, RSR, and RIR, respectively. Given that the difference between each value of RAR was small, two decimal places were reported to ensure a closer approximation to the real situation and better differentiation.
Diagnostic performance of DUS for detecting RAS ≥70%
All the Doppler variables exhibited good accuracy in the diagnosis of severe RAS (83.96–89.62%). The sensitivity, specificity, PPV, NPV, and total accuracy are detailed in Table 2. At a cutoff level of 249.5 cm/s, PSVmain had a specificity of 90.91% and a PPV of 92.59% in the detection of RAS ≥70%. An RAR greater than 2.94 was identified as the optimal cutoff value to detect RAS greater than 70%, with a total accuracy of 86.79%. When RAR was considered as the diagnostic criterion, the sensitivity was better (93.55%), and a comparable accuracy (86.79%) was obtained. An RSR greater than the threshold of 5.1 provided a sensitivity of 93.55%, an NPV of 90.24%, and a total accuracy of 89.62%, with these values being slightly better than those noted for the other parameters. When RIR was considered as the diagnostic criterion, the sensitivity was better than that of PSVmain (88.71% vs. 80.65%), whereas the specificity, PPV, NPV and total accuracy were relatively inferior. Compared with other parameters, RAR and RSR exhibited better sensitivities (93.55%) and total accuracies (86.79% and 89.62%). PSVmain showed better specificity (90.91%) and PPV (92.59%) than did the other Doppler variables.
Table 2
| Doppler variable (thresholds) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | AUC (95% CI) |
|---|---|---|---|---|---|---|
| PSVmain (249.5 cm/s) | 80.65 | 90.91 | 92.59 | 76.92 | 84.91 | 0.858 (0.777–0.918) |
| RAR (2.94) | 93.55 | 77.27 | 85.29 | 89.47 | 86.79 | 0.854 (0.772–0.915) |
| RSR (5.1) | 93.55 | 84.09 | 89.23 | 90.24 | 89.62 | 0.888 (0.812–0.941) |
| RIR (7.5) | 88.71 | 77.27 | 84.62 | 82.93 | 83.96 | 0.830 (0.745–0.896) |
RAS, renal artery stenosis; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval; PSV, peak systolic velocity; PSVmain, PSV of the main renal artery; RAR, ratio of the peak velocities in the renal artery and the aorta; RSR, ratio of the peak velocities in the renal artery and the segmental artery; RIR, ratio of the peak velocities in the renal artery and the interlobar artery.
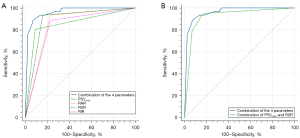
The AUCs of PSVmain, RAR, RSR, and RIR alone were similar and showed no statistically significant difference in pairwise comparisons (Table 3). At a cutoff value of 5.1, the AUC of RSR was 0.888 (95% CI: 0.812–0.941). To improve the accuracy for diagnosis, two or more Doppler variables were combined, and then the AUCs were calculated and compared with either parameter alone (Table 3). The combination of these four Doppler variables (PSVmain, RAR, RSR, and RIR) demonstrated a significant benefit to the overall diagnostic value compared with the above 4 parameters used alone (AUC =0.962; 95% CI: 0.906–0.989; P<0.05) (Figure 2A). Considering that the measurement of all these ultrasound variables is time-consuming and complicated when employed in daily clinical procedures, the basic and easily obtained duplex parameters, PSVmain and RSR, which directly reflect stenosis grading and indirectly indicate intrarenal blood perfusion, were combined to balance the sensitivity and specificity in the diagnosis of severe RAS. Comparison of AUCs showed that the combination of PSVmain and RSR (AUC =0.925; 95% CI: 0.858–0.967) exhibited comparable diagnostic efficiency to the combination of four analyzed ultrasonographic variables in the detection of RAS ≥70% (Figure 2B). No statistically significant difference was observed between these two groups (z statistic =1.882; P=0.06).
Table 3
| Object | z statistic | Significance level (P) |
|---|---|---|
| PSVmain + RAR + RSR + RIR vs. PSVmain | 3.676 | <0.001 |
| PSVmain + RAR + RSR + RIR vs. RAR | 3.727 | <0.001 |
| PSVmain + RAR + RSR + RIR vs. RSR | 2.992 | 0.003 |
| PSVmain + RAR + RSR + RIR vs. RIR | 4.070 | <0.001 |
| PSVmainvs. RAR | 0.078 | 0.94 |
| PSVmainvs. RSR | 0.869 | 0.39 |
| PSVmainvs. RIR | 0.749 | 0.45 |
| RAR vs. RSR | 0.863 | 0.39 |
| RAR vs. RIR | 0.554 | 0.58 |
| RSR vs. RIR | 1.768 | 0.08 |
| PSVmain + RSR vs. PSVmain + RAR + RSR + RIR | 1.882 | 0.06 |
ROC, receiver operating characteristic; PSV, peak systolic velocity; PSVmain, PSV of the main renal artery; RAR, ratio of the peak velocities in the renal artery and the aorta; RSR, ratio of the peak velocities in the renal artery and the segmental artery; RIR, ratio of the peak velocities in the renal artery and the interlobar artery.
Discussion
RAS is a common cause of secondary hypertension, especially in older adult patients with other atherosclerotic manifestations, and is frequently associated with refractory hypertension and renal insufficiency (28,29). Zierler et al. observed the natural history of RAS in patients who were not candidates for immediate renal revascularization and found that the degree of RAS progressed every year during the study (30). Therefore, precise assessment of RAS, especially severe RAS, is essential to appropriately directing clinical decision-making (31-33).
RAS that can be surgically interfered with is typically located in the main renal artery. Ultrasonography is recommended as the first-line method for screening RAS, and routine use of DUS is encouraged for detecting silent atherosclerotic lesions in the renal artery in clinical practice (2,28). The majority of previous studies defined RAS greater than 60% as severe RAS (2,20,21,26-28). However, based on our experience, these clinical justification criteria may overestimate the impact of RAS, resulting in unnecessary clinical intervention. In this study, diameter reduction ≥70% was defined as severe stenosis, which was similar to the evaluation criteria of DSA, and could accurately identify patients as potential candidates for angiography or revascularization.
Baxter et al. reported that color DUS failed to demonstrate a stenotic segment of the renal artery in 16% cases (34). CEUS can better display the entire main trunk of the bilateral renal arteries than can color Doppler flow imaging (CDFI) and is remarkably consistent with DSA in the diagnosis of RAS ≥70% (17). We used CEUS to display the stenosis of the main renal artery, which provide clearer and more accurate visualization than did the CDFI images used in early studies. Accurate display of the main RAS site is the basis of the accurate measurement of stenosis velocity.
Renal artery CEUS is complicated and time-consuming (15 to 60 minutes) and requires that examiners have considerable experience and professional training in abdominal and vascular ultrasonography (35). In basic-level hospitals, DUS remains the leading method for diagnosing RAS. Therefore, in this study, the point was to identify DUS hemodynamic parameters that can be simply acquired by most sonographers and identify their optimal thresholds for accurately diagnosing severe RAS.
The most frequently used parameter of DUS is PSV, which depends on a direct evaluation of elevated velocity in a stenosed segment (1). The results of a meta-analysis demonstrated that PSV had the highest performance characteristics compared with other parameters such as RAR, with an expected sensitivity and specificity of 85% and of 92%, respectively (36). Once the RAS waveform is obtained, the measurement of the PSV is simpler and more accurate. In addition, a higher velocity may correlate with a greater pressure differential across the stenosis (3). Therefore, in this study, we chose PSV as the fundamental parameter to investigate a SAFE method for diagnosing RAS ≥70%. However, PSV is influenced by current blood pressure, wall vessel compliance, the tortuosity of renal arteries, and chronic renal parenchymal damage, and in young people, it can also be affected by hyperdynamic circulation, hyperthyroidism, and anemia (18). Therefore, the ratio of PSV (RAR, RSR, and RIR) was used together to decrease the influence of the abovementioned systemic factors on PSV and produce more reliable results.
The mean PSVmain of the RAS ≥70% was 312.2±82.8 cm/s in this study, which is similar to the results reported by Krumme et al. (316±146 cm/s) (22). The suggested PSVmain threshold values vary among studies, and a PSV of 200 cm/s is the commonly reported cutoff value in the diagnosis of RAS ≥60% (1,7,8,20,28). In this study, we selected the same criteria as those of DSA for the diagnosis of severe RAS (diameter reduction ≥70%). The optimal threshold of PSV was 249.5 cm/s. With this cutoff value, PSVmain showed good specificity and PPV (90.91% and 92.59%, respectively). Some authors recommend a higher PSV threshold of 300 cm/s to improve specificity (1), but based on our analysis, this would significantly reduce the total accuracy. In addition, RAS is often located in the ostial position, and there can be potential challenges related to angle problems. Due to the anatomy and positioning, the angle at which the DUS beam encounters the vessels can affect the measured flow velocity. The blood flow spectrum measured in the supine position (used in most studies) may be higher than the actual value, leading to an overestimation of the degree of RAS. In this study, we adjusted the probe and patient position to optimize the angle of insonation and reduce resulting measurement errors. A greater than 249.5 cm/s increase in PSV can accurately diagnosis severe RAS.
PSV ratios are minimally affected by the above factors that affect the PSV result; therefore, it is both necessary and feasible to measure PSVmain, RAR, RIR, or RSR in the diagnosis of RAS (26). The ratios of PSV (RAR, RSR, and RIR) demonstrated better sensitivity and NPV than did PSVmain alone in predicting RAS ≥70% with the thresholds. The RSR showed slightly better overall accuracy (89.62%) than did the other Doppler parameters. Li et al. reported comparable results also based on Asian populations (26). The optimal threshold values of PSVmain, RAR, RSR, and RIR were 170 cm/s, 2.3, 4.0, and 5.5, respectively, for the diagnosis of RAS ≥50% (26). In this study, a greater than 249.5 cm/s increase in PSVmain, a greater than 2.94 increase in RAR, a greater than 5.1 increase in RSR, and a greater than 7.5 increase in RIR represent more realistic diagnostic cutoff levels for the diagnosis of RAS ≥70% and will help select patients who should proceed to angiography and revascularization.
These ultrasound Doppler hemodynamic parameters (PSV and PSV ratios) show good diagnostic performance in the recognition of severe RAS, and no significant difference was noted among these parameters.
The combination of PSVmain, RAR, RSR, and RIR demonstrated significantly better diagnostic efficiency than any one of them alone in predicting RAS ≥70% (P<0.05). The AUC of the combined parameters was 0.962 (95% CI: 0.906–0.989). In routine clinical practice, the recognition and measurement of the peak velocities in the segmental artery is much easier and more accurate than other Doppler parameters. To simplify its clinical application, two Doppler parameters (PSVmain and RSR) were combined and showed comparable diagnostic performance to the combination of 4 parameters (PSVmain, RAR, RSR, and RIR) (AUC =0.925; 95% CI: 0.858–0.967; z statistic =1.882; P=0.06). These simple DUS hemodynamic parameters can accurately diagnose severe RAS.
In contrast to previous studies, we used modified lateral lumbar coronal sections to visualize the main renal artery and measure the blood flow spectrum of the stenosis. The maximal Doppler shift occurs at an angle (θ) of 0°. A 5° error in estimating the Doppler angle at 30° will cause a 5.4% error in velocity determination (37). Therefore, the best signal and best spectral image are obtained when the direction of flow is parallel to the ultrasound beam (38). Measuring the PSV in the modified section has the advantage of the flow velocity being closer to the actual flow velocity at the stenosis, which may prevent the overestimation of stenosis, reflecting the actual hemodynamic changes and providing results comparable to angiography.
This study had several limitations. First, as we employed a retrospective design, bias in patient selection and validation was inevitable. Moreover, the majority of patients included in the study were older adults, which may limit its application value to other groups. Second, stenosis of anomalous renal arteries, such as accessory RAS, was not included, which might have led to a decrease in the sensitivity of the application of this result in clinical practice. Third, obesity, bowel gas, and dense atherosclerotic plaques may interfere with the display of the main renal artery. In this situation, the velocity of the stenosis cannot be detected.
Conclusions
We constructed a simple method to predict RAS ≥70% based on the PSV and PSV ratios using basic DUS. This simple and accurate method has the potential to facilitate the detection of severe RAS in the majority of medical institutions, especially in basic-level hospitals, and to provide a reliable basis for the selection of suitable candidates for further angiography or revascularization.
Acknowledgments
We are grateful to the surgeons, radiologists, and technicians for their valuable contributions to this study.
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-605/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-605/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Beijing Hospital (No. 2018BJYYEC-043-02). All patients or their legal guardians signed an informed consent form before examination.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harvin HJ, Verma N, Nikolaidis P, Hanley M, Dogra VS, Goldfarb S, Gore JL, Savage SJ, Steigner ML, Strax R, Taffel MT, Wong-You-Cheong JJ, Yoo DC, Remer EM, Dill KE, Lockhart ME. ACR Appropriateness Criteria(®) Renovascular Hypertension. J Am Coll Radiol 2017;14:S540-9. [Crossref] [PubMed]
- Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763-816. [Crossref] [PubMed]
- Dworkin LD, Cooper CJ. Clinical practice. Renal-artery stenosis. N Engl J Med 2009;361:1972-8. [Crossref] [PubMed]
- Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009;361:1953-62. [Crossref] [PubMed]
- Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr, Dworkin LD. CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014;370:13-22. [Crossref] [PubMed]
- Murphy TP, Cooper CJ, Matsumoto AH, Cutlip DE, Pencina KM, Jamerson K, Tuttle KR, Shapiro JI, D’Agostino R, Massaro J, Henrich W, Dworkin LD. Renal Artery Stent Outcomes: Effect of Baseline Blood Pressure, Stenosis Severity, and Translesion Pressure Gradient. J Am Coll Cardiol 2015;66:2487-94. [Crossref] [PubMed]
- Tafur JD, White CJ. Renal Artery Stenosis: When to Revascularize in 2017. Curr Probl Cardiol 2017;42:110-35. [Crossref] [PubMed]
- Zeller T, Bonvini RF, Sixt S. Color-coded duplex ultrasound for diagnosis of renal artery stenosis and as follow-up examination after revascularization. Catheter Cardiovasc Interv 2008;71:995-9. [Crossref] [PubMed]
- Mazloumi M, Van Gompel G, Kersemans V, de Mey J, Buls N. The presence of contrast agent increases organ radiation dose in contrast-enhanced CT. Eur Radiol 2021;31:7540-9. [Crossref] [PubMed]
- Tsai LL, Grant AK, Mortele KJ, Kung JW, Smith MP. A Practical Guide to MR Imaging Safety: What Radiologists Need to Know. Radiographics 2015;35:1722-37. [Crossref] [PubMed]
- Dewey M, Schink T, Dewey CF. Claustrophobia during magnetic resonance imaging: cohort study in over 55,000 patients. J Magn Reson Imaging 2007;26:1322-7. [Crossref] [PubMed]
- Contrast Media Safety Committee. (2022) ESUR guidelines on contrast agents,version 10.0. Available online: https://www.esur.org/esur-guidelines-on-contrast-agents/, accessed 28 Nov 2022.
- American College of Radiology. (2022) Manual on contrast media, version 10.3. Available online: https://www.acr.org/Clinical-Resources/Contrast-Manual, accessed 28 Nov 2022.
- Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, McDonald RJ, Rodby RA, Wang CL, Weinreb JC. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020;294:660-8. [Crossref] [PubMed]
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, Golzarian J, Gornik HL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler REAmerican College of Cardiology Foundation Task Force. American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:1555-70. [Crossref] [PubMed]
- Huang DY, Yusuf GT, Daneshi M, Ramnarine R, Deganello A, Sellars ME, Sidhu PS. Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol (NY) 2018;43:960-76. [Crossref] [PubMed]
- Wang Y, Li Y, Wang S, Ma N, Ren J. Role of Contrast-Enhanced Ultrasound in the Evaluation of Patients With Suspected Renal Arterial Stenosis. Front Cardiovasc Med 2022;9:721201. [Crossref] [PubMed]
- Boddi M. Renal Ultrasound (and Doppler Sonography) in Hypertension: An Update. Adv Exp Med Biol 2017;956:191-208. [Crossref] [PubMed]
- AIUM Practice Parameter for the Performance of Duplex Sonography of Native Renal Vessels. J Ultrasound Med 2020;39:E24-9. [Crossref] [PubMed]
- Miralles M, Cairols M, Cotillas J, Giménez A, Santiso A. Value of Doppler parameters in the diagnosis of renal artery stenosis. J Vasc Surg 1996;23:428-35. [Crossref] [PubMed]
- Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med 1995;122:833-8. [Crossref] [PubMed]
- Krumme B, Blum U, Schwertfeger E, Flügel P, Höllstin F, Schollmeyer P, Rump LC. Diagnosis of renovascular disease by intra- and extrarenal Doppler scanning. Kidney Int 1996;50:1288-92. [Crossref] [PubMed]
- Ripollés T, Aliaga R, Morote V, Lonjedo E, Delgado F, Martínez MJ, Vilar J. Utility of intrarenal Doppler ultrasound in the diagnosis of renal artery stenosis. Eur J Radiol 2001;40:54-63. [Crossref] [PubMed]
- Patriquin HB, Lafortune M, Jéquier JC, O’Regan S, Garel L, Landriault J, Fontaine A, Filiatrault D. Stenosis of the renal artery: assessment of slowed systole in the downstream circulation with Doppler sonography. Radiology 1992;184:479-85. [Crossref] [PubMed]
- Schwerk WB, Restrepo IK, Stellwaag M, Klose KJ, Schade-Brittinger C. Renal artery stenosis: grading with image-directed Doppler US evaluation of renal resistive index. Radiology 1994;190:785-90. [Crossref] [PubMed]
- Li JC, Jiang YX, Zhang SY, Wang L, Ouyang YS, Qi ZH. Evaluation of renal artery stenosis with hemodynamic parameters of Doppler sonography. J Vasc Surg 2008;48:323-8. [Crossref] [PubMed]
- AbuRahma AF, Srivastava M, Mousa AY, Dearing DD, Hass SM, Campbell JR, Dean LS, Stone PA, Keiffer T. Critical analysis of renal duplex ultrasound parameters in detecting significant renal artery stenosis. J Vasc Surg 2012;56:1052-9, 1060.e1; discussion 1059-60.
- Battaglia Y, Fiorini F, Gisonni P, Imbriaco M, Lentini P, Zeiler M, Russo L, Prencipe M, Russo DUltrasound Study Group of the Italian Society of Nephrology. Ultrasonographic Assessment of Atherosclerotic Renal Artery Stenosis in Elderly Patients with Chronic Kidney Disease: An Italian Cohort Study. Diagnostics (Basel) 2022;12:1454. [Crossref] [PubMed]
- Safian RD. Renal artery stenosis. Prog Cardiovasc Dis 2021;65:60-70. [Crossref] [PubMed]
- Zierler RE, Bergelin RO, Isaacson JA, Strandness DE Jr. Natural history of atherosclerotic renal artery stenosis: a prospective study with duplex ultrasonography. J Vasc Surg 1994;19:250-7; discussion 257-8. [Crossref] [PubMed]
- Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 2001;344:431-42. [Crossref] [PubMed]
- Bhalla V, Textor SC, Beckman JA, Casanegra AI, Cooper CJ, Kim ESH, Luther JM, Misra S, Oderich GSAmerican Heart Association Council on the Kidney in Cardiovascular Disease. Council on Hypertension; Council on Peripheral Vascular Disease; and Council on Cardiovascular Radiology and Intervention. Revascularization for Renovascular Disease: A Scientific Statement From the American Heart Association. Hypertension 2022;79:e128-43. [Crossref] [PubMed]
- Savard S, Steichen O, Azarine A, Azizi M, Jeunemaitre X, Plouin PF. Association between 2 angiographic subtypes of renal artery fibromuscular dysplasia and clinical characteristics. Circulation 2012;126:3062-9. [Crossref] [PubMed]
- Baxter GM, Aitchison F, Sheppard D, Moss JG, McLeod MJ, Harden PN, Love JG, Robertson M, Taylor G. Colour Doppler ultrasound in renal artery stenosis: intrarenal waveform analysis. Br J Radiol 1996;69:810-5. [Crossref] [PubMed]
- Halpern EJ, Needleman L, Nack TL, East SA. Renal artery stenosis: should we study the main renal artery or segmental vessels? Radiology 1995;195:799-804. [Crossref] [PubMed]
- Williams GJ, Macaskill P, Chan SF, Karplus TE, Yung W, Hodson EM, Craig JC. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: paired and unpaired analysis. AJR Am J Roentgenol 2007;188:798-811. [Crossref] [PubMed]
- Logason K, Bärlin T, Jonsson ML, Boström A, Hårdemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50-69% and 70-99% carotid artery stenosis. Eur J Vasc Endovasc Surg 2001;21:311-3. [Crossref] [PubMed]
- Revzin MV, Imanzadeh A, Menias C, Pourjabbar S, Mustafa A, Nezami N, Spektor M, Pellerito JS. Optimizing Image Quality When Evaluating Blood Flow at Doppler US: A Tutorial. Radiographics 2019;39:1501-23. [Crossref] [PubMed]


