
Advantages of computed tomography-based navigation in clipping distal anterior cerebral artery aneurysms: a retrospective cohort study
Introduction
Distal anterior cerebral artery (DACA) aneurysms account for 2–9% of all intracranial aneurysms. They are usually smaller and more likely to rupture than are aneurysms in other locations (1-4). Ruptured DACA aneurysms cause subarachnoid hemorrhage and may also lead to hemorrhage in the frontal lobe parenchyma or hematoma of the corpus callosum (5). Surgical clipping of DACA aneurysms is challenging because of their small size, deep location, and adhesion to the frontal lobe in a narrow longitudinal fissure (6-8). Endovascular treatment is not suitable for ruptured DACA aneurysms in most cases because the parent artery of DACA aneurysms is small, and most DACA aneurysms are wide-neck aneurysms that require stent-assisted embolization followed by double antiplatelet therapy, which may increase the risk of rebleeding. Most ruptured DACA aneurysms are treated with surgical clipping, for which a considerable amount of time is spent in locating aneurysms because of their deep location (except for a few superficial aneurysms), increasing the time of surgery and amount of anesthesia (9). Neuronavigation based on computed tomography (CT) or magnetic resonance imaging can accurately locate intracranial lesions, which can help decrease the damage to tissue and reduce the operation time. Although neuronavigation has been used for over a decade (10,11), the efficacy and safety of surgical clipping of DACA aneurysms with the assistance of navigation has not yet been confirmed (12-14). Therefore, we aimed to investigate whether neuronavigation has an advantage in certain surgical procedures by comparing the outcomes of patients in a navigation group with those in a traditional non-navigation group. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-671/rc).
Methods
Patients
From January 2013 to November 2021, the data of 164 patients were retrieved from the Department of Neurosurgery, Renmin Hospital of Wuhan University. Due to the low incidence of DACA aneurysms, we collected as many cases as possible that met the criteria. A total of 139 patients were enrolled in this study, including 36 patients who underwent clipping with the assistance of neuronavigation and 103 who underwent traditional microsurgery without navigation (Figure 1). All patients were diagnosed with aneurysmal subarachnoid hemorrhage through CT and CT angiography (CTA). Patients were divided into two groups based on whether they received intraoperative navigational assistance during aneurysm clipping. Inclusion criteria for this study required that patients were definitively diagnosed with DACA aneurysms after CTA or digital subtraction angiography (DSA), had complete clinical data, and underwent craniotomy to clip the intracranial aneurysm. Exclusion criteria included recurrence of intracranial aneurysms, history of traumatic brain injury or brain surgery, blood disease or recent anticoagulant use, and severe organ dysfunction. As this was a retrospective study, grouping was based solely on whether neuronavigation was used during surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was reviewed and approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2021-K070). Individual consent for this retrospective analysis was waived.
Data on gender, age, Hunt-Hess grade, Fisher grade, modified Rankin Scale (mRS) score, aneurysm location, hospitalization time, aneurysm found time (the duration from incision to aneurysm discovery), and intraoperative bleeding volume were collected from medical records and neurosurgical databases. Two experienced neurologists performed the clinical evaluations and follow-up assessments. For the clinical evaluation, the length of stay was calculated from date of admission to discharge. The aneurysm found time was calculated from the time of incising the scalp to the time the aneurysm was found during the operation. Intraoperative bleeding volume was defined as the total amount of fluid collected by the blood recovery machine minus the amount of the non-blood fluid in the machine. Non-blood fluids included saline to irrigate the surgical area and heparin saline added to the blood recovery machine, among others. All aneurysms were confirmed via CT and CTA or DSA in all patients before surgery. The feasibility and safety of neuronavigation in the surgical treatment of DACA aneurysms were evaluated according to the standard protocol. The mRS score was scored based on the status of the patients when discharged or followed up. Patients were followed up in clinic or via telephone by investigators who were not involved in the statistical analysis, with a mean follow-up time of 59.2±13.4 months. We followed up all enrolled patients via telephone in May 2022. Nineteen patients were lost to follow-up due to lack of valid contact information. During follow-up, 4 patients died. Their mRS score was categorized as 5, with the cause of death attributed to either heart disease or stroke.
Neuronavigation system
The Brainlab Navigation System (Munich, Germany) was used in the neuronavigation group. Preoperatively, brain CT and CTA were performed using a Revolution CT Platform (GE HealthCare, Chicago, IL, USA). The interval thickness of each layer of the CT scan image was 0.625 mm. The source image of CTA was used for diagnosis and three-dimensional (3D) reconstruction in the axial, coronal, and sagittal planes with iPlan 3.0 stereotactic software (Brainlab).
Surgical clipping
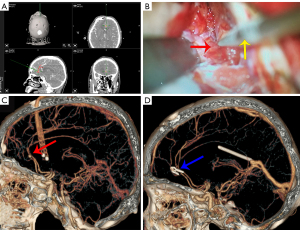
Neuronavigation-assisted surgery was based on CTA in the navigation group. First, the patient was placed in the supine position, and the head was fixed with a Sugita clamp after general anesthesia. The neuronavigation pointer with virtual tip extension was used to mark the upper boundary of the frontal sinus on the patient’s forehead to avoid unnecessary opening during craniotomy. A coronary incision of approximately 5 inches was planned to allow short and straight access to the DACA aneurysms and control of the proximal artery. The operational approach was chosen to avoid damage to the bridge veins. After removal of the bone flap and before opening of the dura mater, a neuronavigation pointer with a virtual tip extension was used to obtain an accurate direction and distance to the aneurysm. Thereafter, the neuronavigation pointer was used for orientation again after the dura was opened before the cerebrospinal fluid was released. During the operation, the tip of the pointer was used to control the exact direction of the target. After the site of the aneurysm was reached through microscopic observation, the accuracy was checked again by directly positioning the tip of the neuronavigation pointer on the aneurysm and comparing its position with the 3D CTA data installed on the navigation screen. This was recorded by intraoperative photos under the microscope and screenshots of the neuronavigation system. In the traditional group, intracranial aneurysms were clipped without neuronavigation. Before discharge, CTA was performed to confirm whether the aneurysm was completely clipped and whether the parent artery was unobstructed. Patients were followed up at the clinic for CTA via the mRS score. Two typical examples are shown in Figures 2,3.

Data analysis
All data were analyzed using SPSS version 26.0 software (IBM Corp., Armonk, NY, USA). According to the results of the previous research group pretest, it was assumed that the average found time detected in the navigation group and traditional group could reach 56 and 63 with a standard deviation of 10. Calculation with PASS 11.0 software (NCSS, Kaysville, UT, USA) indicated that 27 cases should be included in navigation group and 80 in traditional group, with a total of 107 cases in the combined and indirect groups. The parameters are set as follows. The β=0.2, grasp (power = 1 − β) =80%, and a bilateral α of the significance level of 0.05 calculated according to the shedding rate of 20%. Categorical variables are described as numbers or percentages. Continuous variables are expressed as mean ± standard deviation. The differences between the two groups were analyzed using the χ2-test for categorical data and the Student t-test for continuous data. Since the sample size was less than 40, the Fisher exact probability method was selected. Non-parametric tests were used for the hierarchical data. All statistical tests were two-sided, and significance was set at P<0.05, along with 95% confidence intervals.
Results
Baseline characteristics
The patients’ demographic and clinical characteristics are shown in Table 1. Ultimately, 139 patients with DACA aneurysms were enrolled in this study. Among these patients, 91 were females, 72 patients had aneurysms located at the distal A2 segment of the anterior cerebral artery, 55 had aneurysms at the distal A3 segment, and 12 had aneurysms at the distal A4 segment. Except for 2 patients with unruptured aneurysms, all patients had ruptured aneurysms with subarachnoid hemorrhage. The two groups were well balanced for anthropometric characteristics, including age (P=0.644) and gender (P=0.817). No differences were found in the Hunt-Hess grade (P=0.572) or Fisher grade (P=0.561) between the two groups.
Table 1
| Patient characteristics | Navigation group (n=36) | Traditional group (n=103) | Statistical data | |
|---|---|---|---|---|
| χ2 or t | P value | |||
| Age (years) | 56.17±8.31 | 55.32±9.79 | t=0.463 | 0.644 |
| Gender | χ2=0.054 | 0.841 | ||
| Male | 13 | 35 | ||
| Female | 23 | 68 | ||
| Fisher grade | χ2=2.002 | 0.561 | ||
| I | 13 | 25 | ||
| II | 13 | 47 | ||
| III | 4 | 14 | ||
| IV | 6 | 17 | ||
| Hunt-Hess grade | χ2=4.270 | 0.511 | ||
| 0 | 7 | 24 | ||
| I | 13 | 39 | ||
| II | 6 | 18 | ||
| III | 3 | 13 | ||
| IV | 6 | 6 | ||
| V | 1 | 3 | ||
| Aneurysm location | χ2=4.140 | 0.126 | ||
| A2 | 18 | 54 | ||
| A3 | 12 | 43 | ||
| A4 | 6 | 6 | ||
The grade data were analyzed by non-parametric tests. The age data are presented as mean ± standard deviation, while the other data are presented as numbers.
Clinical outcomes
The clinical outcomes are summarized in Table 2. No differences were found for hospitalization between the navigation group and the traditional group {17.5 [16–21] vs. 18 [15–22] days, P=0.761}. The aneurysm found time {49 [42–53] vs. 79 [63–84] min, P<0.001} and intraoperative bleeding volume {370 [280–460] vs. 430 [310–610] mL, P=0.045} in the navigation group were both significantly lower than those in the traditional group. No significant difference was noted in the mRS scores in the long-term follow-up between the two groups (P=0.157), and the mean follow-up time was 42.6±21.0 months.
Table 2
| Patient characteristics | Navigation group (n=36) | Traditional group (n=103) | Statistical data | |
|---|---|---|---|---|
| χ2 | P value | |||
| Hospitalization time (days) | 17.5 [16–21] | 18 [15–22] | – | 0.761 |
| The aneurysm found time (min) | 49 [42–53] | 79 [63–84] | – | <0.001* |
| Intraoperative bleeding volume (mL) | 370 [280–460] | 430 [310–610] | – | 0.045* |
| mRS at discharge | 6.187 | 0.288 | ||
| Grade 0 | 7 | 16 | ||
| Grade 1 | 10 | 42 | ||
| Grade 2 | 7 | 16 | ||
| Grade 3 | 6 | 23 | ||
| Grade 4 | 3 | 4 | ||
| Grade 5 | 3 | 2 | ||
| mRS of long-term follow-up† | 3.443 | 0.487 | ||
| Grade 0 | 21 | 51 | ||
| Grade 1 | 6 | 16 | ||
| Grade 2 | 4 | 12 | ||
| Grade 3 | 0 | 6 | ||
| Grade 4 | 0 | 0 | ||
| Grade 5 | 2 | 2 | ||
*, P<0.05 indicates a statistical difference between the traditional group and the navigation group; †, 19 patients were lost to follow-up. The grade data were analyzed with non-parametric tests. The measurement data were analyzed with the t-test when they were in accordance with normal distribution. The mRS data are presented as numbers, while the other data are presented as mean [interquartile range]. mRS, modified Rankin Scale.
Discussion
Our study suggests that clipping DACA aneurysms through neuronavigation may reduce intraoperative bleeding volume and shorten the time to find aneurysms although no significant difference was found in the long-term prognosis. We found that neuronavigation can also significantly reduce the time to reach the aneurysm to shorten the operation time, thus assuaging the operators concerns and providing peace of mind in performing subsequent clipping. Using CTA neuronavigation is helpful and safe for the surgical treatment of DACA aneurysms although these aneurysms presents special challenges compared with other anterior circulation aneurysms (2,3,6,8,15,16).
Difficulties of clipping and endovascular treatment
DACA aneurysms are located near the corpus callosum and deeper into the midline between both hemispheres, which are difficult to expose because the anterior longitudinal fissure is smaller than is the Sylvian cistern. Reaching these aneurysms during surgery requires sufficient exposure in the anterior longitudinal fissure (2,15), thus undoubtedly increasing the time of dissection and the possibility of brain damage. Even for an experienced surgeon, there are various challenges in treating aneurysms at these locations (4,6). It is well known that most DACA aneurysms are small, with a wide neck and a thin dome, which makes them difficult to clip (2,4,15). Retracting the frontal lobe during surgery is needed to gain access to the aneurysm after subarachnoid and cerebral hemorrhage. Bridge vein injury and subsequent vein infarction and intraoperative aneurysm rupture are possible complications (4,17,18).
Although endovascular treatment can be used to manage almost all intracranial aneurysms, interventional treatment of DACA aneurysms remains a challenge because of the long route from the aortic arch and the thin feeding artery (4,6). Moreover, endovascular treatment is unable to remove the hematoma and reduce intracranial pressure. For some scenarios, open surgery remains an alternative method in treating DACA aneurysms.
Development of neuronavigation in neurosurgery
Neuronavigation has become a powerful tool in a variety of operations (19), as it allows surgeons to obtain precise access to the lesion, especially for deep small lesions in the cerebral parenchyma (20). During the last 20 years, neuronavigation has become an increasingly popular neurosurgical tool for minimizing invasiveness and maximizing safety (21). Neuronavigation is mostly based on CTA or MRA data. CTA is a noninvasive and fast imaging method that shows the same sensitivity and specificity as that of DSA in aneurysms larger than 2 mm. With CTA, navigation can be used to outline not only pathological changes but also peripheral blood vessels and nerve tissue that must be foreseen, recognized, and preserved (22). Good results are also reported when unnecessary retraction and cortical damage are avoided during the operation.
Advantages and risks of neuronavigation
Studies have shown that using neuronavigation to locate unruptured aneurysms can minimize the incidence of surgical complications in clipping intracranial aneurysm (23,24). In this study, we found that the operating time was also shortened in ruptured DACA aneurysms with the assistance of neuronavigation. The postoperative length of hospital stay was significantly shortened in the neuronavigation group, which could be attributed to the unnecessary retraction and cortical damage that were avoided during the operation.
The reported disadvantage of neuronavigation is an increased risk of postoperative infection, which is accompanied by a significant increase in surgical costs (25). Moreover, the brain drift during surgery for DACA aneurysms may influence the accuracy and disrupt surgical planning (26).
Advanced neuronavigation technology
To avoid deviation in navigation, intraoperative ultrasound can reduce errors that may result from changes in aneurysm location after CSF drainage or hematoma removal (25). Augmented reality (AR) also allows data from the real world to be combined with virtual information, making neurosurgery more viable (27). During the procedure, the neuronavigation system can be connected to a surgical microscope, and a transparent computer-generated vascular tree reconstruction can be displayed in the microscope eyepiece (22,28). Virtual reality (VR) and AR have shown advantages in preoperative planning and multimodal neuronavigation for spinal and brain surgery. Furthermore, studies indicate that VR and AR have beneficial effects on medical education and neurosurgery training (29). Intraoperative AR has great potential in helping surgeons identify tumor locations, delineate planned dissection planes, and reduce the risk of damage to nonvisible structures (30). There are several advantages to using the microscope focus as a virtual pointer in microscopic brain tumor surgery, the main one being the elimination of the need to switch to a real pointer (31).
In our study, we precisely oriented all the aneurysms in part by using a navigation pointer before opening the dura, which decreased the drift of brain tissue and the aneurysm. However, this study included only a small sample of patients, and the patients were from a single center. Another limitation of this study is the variable duration of follow-up intervals among each patient, which could have influenced the follow-up outcomes. This means that prospective multicenter large-sample studies are needed to confirm the findings of this study.
Conclusions
Clipping of DACA aneurysms with the assistance of CT neuronavigation may improve surgical accuracy, reduce intraoperative bleeding volume, and shorten the time to finding aneurysms. Neurosurgeons can decide whether to use neuronavigation assistance when clipping DACA aneurysms based on their own surgical experience, patient condition, and financial situation. Naturally, CT navigation can be improved, and in the future, more advanced technologies, such as AR and VR, may be applied in neurosurgery to assist neurosurgeons in performing surgery more quickly and efficiently.
Acknowledgments
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-671/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-671/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was reviewed and approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2021-K070). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoz SS. Intracranial Aneurysms. In: Vascular Neurosurgery. Cham: Springer; 2017.
- Lehecka M, Dashti R, Hernesniemi J, Niemelä M, Koivisto T, Ronkainen A, Rinne J, Jääskeläinen J. Microneurosurgical management of aneurysms at the A2 segment of anterior cerebral artery (proximal pericallosal artery) and its frontobasal branches. Surg Neurol 2008;70:232-46; discussion 246. [Crossref] [PubMed]
- Pandey A, Rosenwasser RH, Veznedaroglu E. Management of distal anterior cerebral artery aneurysms: a single institution retrospective analysis (1997-2005). Neurosurgery 2007;61:909-16; discussion 916-7. [Crossref] [PubMed]
- Rao BR. Surgery for distal anterior cerebral artery aneurysm. Neurol India 2016;64:1145-6. [Crossref] [PubMed]
- Heit JJ, Iv M, Wintermark M. Imaging of Intracranial Hemorrhage. J Stroke 2017;19:11-27. [Crossref] [PubMed]
- Menovsky T, van Rooij WJ, Sluzewski M, Wijnalda D. Coiling of ruptured pericallosal artery aneurysms. Neurosurgery 2002;50:11-4; discussion 14-5. [Crossref] [PubMed]
- Zhou J, Wang Y, Wang D, Chen Q, Wang H, Gao L. Endovascular Treatment for Ruptured Aneurysms at Distal Cerebral Arteries. World Neurosurg 2019;123:e387-92. [Crossref] [PubMed]
- Shukla D, Bhat DI, Srinivas D, Somanna S, Pandey P, Chandramouli BA, Sastry KV, Das BS. Microsurgical treatment of distal anterior cerebral artery aneurysms: A 25 year institutional experience. Neurol India 2016;64:1204-9. [Crossref] [PubMed]
- Monroy-Sosa A, Nathal E, Rhoton AL Jr. Operative Management of Distal Anterior Cerebral Artery Aneurysms Through a Mini Anterior Interhemispheric Approach. World Neurosurg 2017;108:519-28. [Crossref] [PubMed]
- Marmulla R, Hilbert M, Niederdellmann H. Intraoperative precision of mechanical, electromagnetic, infrared and laser-guided navigation systems in computer-assisted surgery. Mund Kiefer Gesichtschir 1998;2:S145-8. [Crossref] [PubMed]
- Suess O, Kombos T, Kurth R, Suess S, Mularski S, Hammersen S, Brock M. Intracranial image-guided neurosurgery: experience with a new electromagnetic navigation system. Acta Neurochir (Wien) 2001;143:927-34. [Crossref] [PubMed]
- Hayhurst C, Beems T, Jenkinson MD, Byrne P, Clark S, Kandasamy J, Goodden J, Nandoe Tewarie RD, Mallucci CL. Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. J Neurosurg 2010;113:1273-8. [Crossref] [PubMed]
- Wu R, Qin H, Cai Z, Shi J, Cao J, Mao Y, Dong B. The Clinical Efficacy of Electromagnetic Navigation-Guided Hematoma Puncture Drainage in Patients with Hypertensive Basal Ganglia Hemorrhage. World Neurosurg 2018;118:e115-22. [Crossref] [PubMed]
- Kandasamy J, Hayhurst C, Clark S, Jenkinson MD, Byrne P, Karabatsou K, Mallucci CL. Electromagnetic stereotactic ventriculoperitoneal csf shunting for idiopathic intracranial hypertension: a successful step forward? World Neurosurg 2011;75:155-60; discussion 32-3.
- Lehecka M, Lehto H, Niemelä M, Juvela S, Dashti R, Koivisto T, Ronkainen A, Rinne J, Jääskeläinen JE, Hernesniemi JA. Distal anterior cerebral artery aneurysms: treatment and outcome analysis of 501 patients. Neurosurgery 2008;62:590-601; discussion 590-601. [Crossref] [PubMed]
- de Sousa AA, Dantas FL, de Cardoso GT, Costa BS. Distal anterior cerebral artery aneurysms. Surg Neurol 1999;52:128-35; discussion 135-6. [Crossref] [PubMed]
- Inci S, Erbengi A, Ozgen T. Aneurysms of the distal anterior cerebral artery: report of 14 cases and a review of the literature. Surg Neurol 1998;50:130-9; discussion 139-40. [Crossref] [PubMed]
- Park J, Hamm IS. Anterior interhemispheric approach for distal anterior cerebral artery aneurysm surgery: preoperative analysis of the venous anatomy can help to avoid venous infarction. Acta Neurochir (Wien) 2004;146:973-7; discussion 977.
- Shamir RR, Freiman M, Joskowicz L, Spektor S, Shoshan Y. Surface-based facial scan registration in neuronavigation procedures: a clinical study. J Neurosurg 2009;111:1201-6.
- Sun GC, Chen XL, Yu XG, Liu G, Xu BN. Paraventricular or centrum ovale cavernous hemangioma involving the pyramidal tract in children: intraoperative MRI and functional neuronavigation-guided resection. Childs Nerv Syst 2015;31:1097-102. [Crossref] [PubMed]
- Inoue D, Cho B, Mori M, Kikkawa Y, Amano T, Nakamizo A, Yoshimoto K, Mizoguchi M, Tomikawa M, Hong J, Hashizume M, Sasaki T. Preliminary study on the clinical application of augmented reality neuronavigation. J Neurol Surg A Cent Eur Neurosurg 2013;74:71-6. [Crossref] [PubMed]
- Meola A, Cutolo F, Carbone M, Cagnazzo F, Ferrari M, Ferrari V. Augmented reality in neurosurgery: a systematic review. Neurosurg Rev 2017;40:537-48. [Crossref] [PubMed]
- Carvalho FG, Godoy BL, Reis M, Gasparetto EL, Wajnberg E, de Souza JM. Frameless stereotactic navigation for intraoperative localization of infectious intracranial aneurysm. Arq Neuropsiquiatr 2009;67:911-3. [Crossref] [PubMed]
- Toyooka T, Otani N, Wada K, Tomiyama A, Takeuchi S, Fujii K, Kumagai K, Fujii T, Mori K. Head-up display may facilitate safe keyhole surgery for cerebral aneurysm clipping. J Neurosurg 2018;129:883-9.
- Onen MR, Yuvruk E, Naderi S, Egemen N. The role of neuronavigation and intraoperative ultrasonography in distal middle cerebral artery aneurysm. Neurosciences (Riyadh) 2018;23:265-7. [Crossref] [PubMed]
- Kil JS, Kim DW, Kang SD. Navigation-guided Keyhole Approach for Unruptured Intracranial Aneurysms. Korean Journal of Cerebrovascular Surgery 2011;13:244-8.
- Mascitelli JR, Schlachter L, Chartrain AG, Oemke H, Gilligan J, Costa AB, Shrivastava RK, Bederson JB. Navigation-Linked Heads-Up Display in Intracranial Surgery: Early Experience. Oper Neurosurg (Hagerstown) 2018;15:184-93. [Crossref] [PubMed]
- Chu Y, Yang J, Ma S, Ai D, Li W, Song H, Li L, Chen D, Chen L, Wang Y. Registration and fusion quantification of augmented reality based nasal endoscopic surgery. Med Image Anal 2017;42:241-56. [Crossref] [PubMed]
- Mishra R, Narayanan MDK, Umana GE, Montemurro N, Chaurasia B, Deora H. Virtual Reality in Neurosurgery: Beyond Neurosurgical Planning. Int J Environ Res Public Health 2022;19:1719. [Crossref] [PubMed]
- Montemurro N, Condino S, Carbone M, Cattari N, D'Amato R, Cutolo F, Ferrari V. Brain Tumor and Augmented Reality: New Technologies for the Future. Int J Environ Res Public Health 2022;19:6347. [Crossref] [PubMed]
- Ammirati M. Augmented reality in brain tumor surgery using the microscope focal point as the virtual pointer. Acta Neurochir (Wien) 2022;164:1. [Crossref] [PubMed]



