
The very low magnetic resonance imaging apparent diffusion coefficient (ADC) measure of abscess is likely due to pus’s specific T2 relaxation time
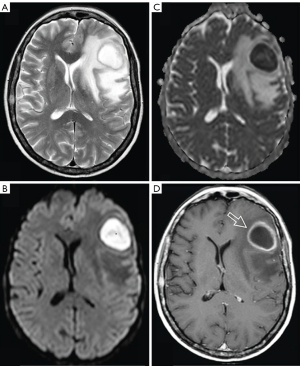
It is well known that abscess fluid (i.e., pus) tends to demonstrate a very low magnetic resonance imaging (MRI)-derived apparent diffusion coefficient (ADC) regardless of the location of the abscess (1-8). At least in the brain, it may appear counterintuitive that abscess pus, being fluid or semi-fluid, has an ADC measure lower than those of white/grey matters (Figure 1).
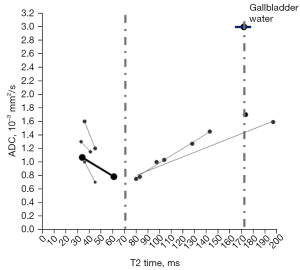
Recently we noted that, regardless of whether b=0 data are included for the ADC calculation, the T2 relaxation time (T2 time) of a tissue or an in vivo substance is strongly associated with ADC measure in many scenarios (9-13) (Figure S1). While ADC measure is affected by many factors, a T2 time of around 70 ms (e.g., from 60 to 80 ms) at 3 T or (or its equivalent values at other magnetic fields) may be associated with the lowest ADC measure (Figure 2). It may be further inferred that, if a tissue (or an in vivo substance) has a T2 time of slightly less than half that of body water (such as gallbladder water: 172 ms at 3 T), then this tissue (or in vivo substance) will have a low ADC measure.
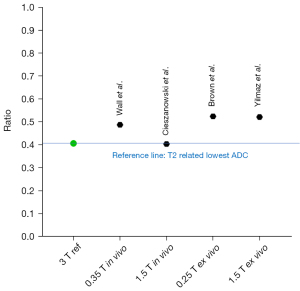
Following the discussion above, we looked at whether abscess pus has a T2 time of slightly less than half that of body water. We were able to identify four studies that reported the T2 times of abscess pus and a body fluid. We took the ratios of ‘abscess pus T2 time/body water T2 time’, and the results are shown in Figure 3 (16-21). It is shown that abscess pus has a T2 time of about half that of body water. Wall et al. reported a T2 time for abscess of 81 ms, and T2 time for muscle, liver and urine of 29, 45 and 166 ms, respectively. The T2 times of the later three are consistent with the other reports at 1.5 or 3.0 T (12,22,23). There is a notion that T2 time doesn’t change much over the range of field strengths used for routine clinical MRI (0.2 to 3.0 T) (23). Additionally, Subhawong et al. (24) described a case series of soft tissue masses, which included one abscess case and three cases of ganglion cyst. T2 signal ratio was measured as ‘lesion T2 weighting signal intensity/muscle T2 weighting signal intensity’. The abscess had a T2 signal ratio of 1.48, while the three ganglion cysts had a mean T2 signal ratio of 3.42, with the abscess’ value being 0.43 of those of the ganglion cysts (abscess ADC: 0.63 mm2/s, ganglion cyst ADC mean: 2.49 mm2/s). The specific T2 relaxation time of pyogenic abscess fluid, according to our viewpoint, contributes to very low ADC measured by MRI. We argue that abscess pus may not have truly restricted diffusion compared with many other in vivo solid tissues. Of course, in real practice, abscess pus composition may vary, and so does its ADC measure (1,4). The discussion in this letter refers to the common scenarios.
Now we look at Figure 1 again. Brain tissues are generally noted to have a short T2 time with grey/dark signal on T2 weighted image. For 3T data, Wansapura et al. (25) described that the average T2 values for occipital and frontal gray matter are 41.6 and 51.8 ms, respectively, and average T2 values for occipital and frontal white matter are 48.4 and 44.7 ms, respectively. According to Figure 3, abscess pus is roughly estimated to have a T2 time of around 80 ms at 3T. Therefore, according to Figure 2, an increase of T2 time from grey/white matter values toward 80 ms would be associated with a lower ADC measure. The abscess high signal shown on Figure 1B (a high b-value diffusion-weighted image) likely does not reflect restricted fluid diffusivity, instead reflects the specific T2 time of the abscess fluid.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1363/coif). YXJW serves as the Editor-in-Chief of Quantitative Imaging in Medicine and Surgery. The author has no other conflicts of interest to declare.
Ethical Statement: The authors is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feraco P, Donner D, Gagliardo C, Leonardi I, Piccinini S, Del Poggio A, Franciosi R, Petralia B, van den Hauwe L. Cerebral abscesses imaging: A practical approach. J Popul Ther Clin Pharmacol 2020;27:e11-24. [Crossref] [PubMed]
- Erdogan C, Hakyemez B, Yildirim N, Parlak M. Brain abscess and cystic brain tumor: discrimination with dynamic susceptibility contrast perfusion-weighted MRI. J Comput Assist Tomogr 2005;29:663-7. [Crossref] [PubMed]
- Chang SC, Lai PH, Chen WL, Weng HH, Ho JT, Wang JS, Chang CY, Pan HB, Yang CF. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging 2002;26:227-36. [Crossref] [PubMed]
- Lotan E, Hoffmann C, Fardman A, Ziv-Baran T, Komisar O, Harnof S. Postoperative versus Spontaneous Intracranial Abscess: Diagnostic Value of the Apparent Diffusion Coefficient for Accurate Assessment. Radiology 2016;281:168-74. [Crossref] [PubMed]
- Chou CP, Chiou SH, Levenson RB, Huang JS, Yang TL, Yu CC, Chiang AJ, Pan HB. Differentiation between pelvic abscesses and pelvic tumors with diffusion-weighted MR imaging: a preliminary study. Clin Imaging 2012;36:532-8. [Crossref] [PubMed]
- Oruç E, Yıldırım N, Topal NB, Kılıçturgay S, Akgöz S, Savcı G. The role of diffusion-weighted MRI in the classification of liver hydatid cysts and differentiation of simple cysts and abscesses from hydatid cysts. Diagn Interv Radiol 2010;16:279-87. [Crossref] [PubMed]
- Oto A, Schmid-Tannwald C, Agrawal G, Kayhan A, Lakadamyali H, Orrin S, Sethi I, Sammet S, Fan X. Diffusion-weighted MR imaging of abdominopelvic abscesses. Emerg Radiol 2011;18:515-24. [Crossref] [PubMed]
- Harish S, Chiavaras MM, Kotnis N, Rebello R. MR imaging of skeletal soft tissue infection: utility of diffusion-weighted imaging in detecting abscess formation. Skeletal Radiol 2011;40:285-94. [Crossref] [PubMed]
- Yu WL, Xiao BH, Ma FZ, Zheng CJ, Tang SN, Wáng YXJ. Underestimation of the spleen perfusion fraction by intravoxel incoherent motion MRI. NMR Biomed 2023;36:e4987. [Crossref] [PubMed]
- Wáng YXJ, Zhao KX, Ma FZ, et al. The contribution of T2 relaxation time to MRI-derived apparent diffusion coefficient (ADC) quantification and its potential clinical implications. Quant Imaging Med Surg 2023;13:7410-6. [Crossref] [PubMed]
- Wang YXJ. Complicated relationships between tissue T2 relaxation time and in vivo tissue diffusion measures, depending on the ranges of T2 value. arXiv:2306.10657. [submitted on 19 Jun 2023]. Available online: https://doi.org/
10.48550 /arXiv.2306.10657 - Wáng YXJ, Ma FZ. A tri-phasic relationship between T2 relaxation time and magnetic resonance imaging (MRI)-derived apparent diffusion coefficient (ADC). Quant Imaging Med Surg 2023; [Crossref]
- Oh J, Cha S, Aiken AH, Han ET, Crane JC, Stainsby JA, Wright GA, Dillon WP, Nelson SJ. Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 2005;21:701-8. [Crossref] [PubMed]
- Mohajeri S, Ijare OB, Bezabeh T, King SB, Thomas MA, Minuk G, Lipschitz J, Kirkpatrick I, Smith M, Smith IC. In vivo 1H MRS of human gallbladder bile at 3 T in one and two dimensions: detection and quantification of major biliary lipids. NMR Biomed 2014;27:1192-202. [Crossref] [PubMed]
- Gajdošík M, Chmelík M, Halilbasic E, Pfleger L, Klepochová R, Trauner M, Trattnig S, Krššák M. In Vivo (1) H MR Spectroscopy of Biliary Components of Human Gallbladder at 7T. J Magn Reson Imaging 2021;53:98-107. [Crossref] [PubMed]
- Wall SD, Fisher MR, Amparo EG, Hricak H, Higgins CB. Magnetic resonance imaging in the evaluation of abscesses. AJR Am J Roentgenol 1985;144:1217-21. [Crossref] [PubMed]
- Brown JJ, vanSonnenberg E, Gerber KH, Strich G, Wittich GR, Slutsky RA. Magnetic resonance relaxation times of percutaneously obtained normal and abnormal body fluids. Radiology 1985;154:727-31. [Crossref] [PubMed]
- Cieszanowski A, Anysz-Grodzicka A, Szeszkowski W, Kaczynski B, Maj E, Gornicka B, Grodzicki M, Grudzinski IP, Stadnik A, Krawczyk M, Rowinski O. Characterization of focal liver lesions using quantitative techniques: comparison of apparent diffusion coefficient values and T2 relaxation times. Eur Radiol 2012;22:2514-24. [Crossref] [PubMed]
- Yilmaz UN, Yaman F, Atilgan SS MR. T1 and T2 relaxations in cysts and abscesses measured by 1.5 T MRI. Dentomaxillofac Radiol 2012;41:385-91. [Crossref] [PubMed]
- Daoust A, Dodd S, Nair G, Bouraoud N, Jacobson S, Walbridge S, Reich DS, Koretsky A. Transverse relaxation of cerebrospinal fluid depends on glucose concentration. Magn Reson Imaging 2017;44:72-81. [Crossref] [PubMed]
- Yoshimura S, Tanaka H, Kawabata S, Kozawa J, Takahashi H, Hidaka Y, Hotta M, Kashiwagi N, Tomiyama N. Effect of urinary glucose concentration and pH on signal intensity in magnetic resonance images. Jpn J Radiol 2022;40:930-8. [Crossref] [PubMed]
- de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 2004;230:652-9. [Crossref] [PubMed]
- Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys 1984;11:425-48. [Crossref] [PubMed]
- Subhawong TK, Durand DJ, Thawait GK, Jacobs MA, Fayad LM. Characterization of soft tissue masses: can quantitative diffusion weighted imaging reliably distinguish cysts from solid masses? Skeletal Radiol 2013;42:1583-92. [Crossref] [PubMed]
- Wansapura JP, Holland SK, Dunn RS, Ball WS Jr. NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging 1999;9:531-8. [Crossref] [PubMed]


