
Comparison of cardiovascular metrics on computed tomography pulmonary angiography of the updated and old diagnostic criteria for pulmonary hypertension in patients with chronic thromboembolic pulmonary hypertension
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a common and important cause of pulmonary hypertension (PH). It is characterized by pulmonary artery occlusion from organized thromboembolic material, causing progressive elevation of pulmonary vascular resistance (PVR) and mean pulmonary artery pressure (mPAP) (1,2). Over time, this pathological process can culminate in right heart failure and even death (1,2). CTEPH can be near-cured with pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA), and medical treatment (1). However, its nonspecific symptoms during the early stage cause a median diagnostic delay of 14 months from the onset of symptoms (3). Computed tomography pulmonary angiography (CTPA) is widely employed for the assessment in CTEPH, and an increased main pulmonary artery diameter (MPAd) and the ratio of MPAd and ascending aorta diameter (MPAd/AAd) along with right heart enlargement on CTPA may indicate the presence of PH (4-18).
In the 2015 European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines, mPAP and PVR for the diagnosis of PH were defined as ≥25 mmHg and ≥3 Wood units (WU), respectively (2,19), a standard that has long been used in clinical work and research. In 2022, the ESC and ERS updated the hemodynamics of PH by lowering the thresholds for mPAP and PVR in healthy individuals to 20 mmHg and 2 WU, respectively (1). This aims to enable patients with suspected PH to receive a timely diagnosis. A meta-analysis reported CTPA had high sensitivity and specificity in the detection of CTEPH when evaluated by expert radiologists (16). However, at present, no research has been conducted to investigate whether the threshold of cardiovascular metrics on CTPA for diagnosing PH is affected by changes in diagnostic criteria. Thus, we aimed to compare cardiovascular metrics on CTPA in the prediction of PH under the updated and old criteria and to reevaluate the metrics that are capable of detecting PH in patients at the early stage of CTEPH. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-250/rc).
Methods
Population and study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Ethics Board of China-Japan Friendship Hospital (No. 2022-KY-048). Individual consent for this retrospective analysis was waived.
For this investigation, 685 participants who underwent right heart catheterization (RHC) between January 2019 and December 2022 were identified from the China-Japan Friendship Hospital. Figure 1 illustrates the process of selecting participants who had CTPA with fully visible lung fields within 1 week after RHC. From this group, 4 cohorts were formed based on the 2015 ESC/ERS guidelines (old) and 2022 ESC/ERS guidelines (updated), and included patients were respectively divided into the following groups: CTEPH patients with mPAP >20 mmHg and control patients with mPAP ≤20 mmHg; and CTEPH patients with mPAP ≥25 mmHg and control patients with mPAP <25 mmHg.

The exclusion criteria were as follows: (I) patients younger than 18 years old or older than 75 years old; (II) patients without CTPA or with poor CTPA quality or incomplete RHC data in our hospital; (III) patients with evidence of chronic obstructive pulmonary disease or interstitial lung; (IV) patients with congenital heart disease or severe cirrhosis; and (V) patients who had undergone PEA before CTPA.
CTPA scan protocol
All patients underwent supine CTPA imaging with either a 256-row CT (GE Revolution CT, GE HealthCare, Chicago, IL, USA) or a 320-row CT (Aquilion ONE, Canon Medical Systems, Otawara, Japan) at the end of expiration, with the lung base to apex being covered. The specific scan parameters for the GE Revolution CT were as follows: a tube rotation speed of 0.28 s/rotation; tube voltage determined via kilovolt (KV) intelligent decision technology (KV assist; 100 and 120 KV), tube current determined via 3D automatic tube current modulation (Smart-milliampere), a pitch of 0.992:1, the slices × collimator width is 256×0.625 mm, and a reconstruction image slice thickness and spacing of 0.625 mm. Meanwhile, the specific scan parameters for the Aquilion ONE were as follows: a tube rotation speed of 0.35 s/rotation, a tube voltage of 120 kVp, a tube current determined via automatic tube current modulation, the Slices × collimator width is 320×0.5 mm, and a reconstruction image slice thickness and spacing of 0.5 mm.
A high-pressure injector was used to administer either iodinated contrast agents (350 mg I/mL) or iodopropamide (370 mg I/mL) through the antebrachial vein. A double-barrel syringe containing contrast agent and saline solution was used, with a flow rate of 5 mL/s, a total contrast agent volume of 60–75 mL, and 30 mL of saline solution. The contrast agent detection method used was automatically triggered, with a trigger threshold of 80 Hounsfield units (HU) in the main pulmonary artery.
RHC
All patients underwent RHC through the right internal jugular or femoral vein with a 6F Swan-Ganz thermodilution catheter (Bioptimal International, Tokyo, Japan). Hemodynamic data, including mPAP, ratio of systolic to diastolic PAP (sPAP/dPAP), mean right atrial pressure (mRAP), mean pulmonary artery wedge pressure (PAWP), ratio of systolic to diastolic blood pressure (sBP/dBP), cardiac output (CO), and cardiac index were obtained during the procedure. PVR was calculated using the following formula (20): PVR = (mPAP – mean PAWP)/CO WU.
Image analysis
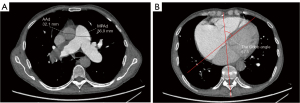
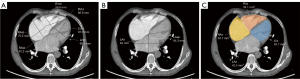
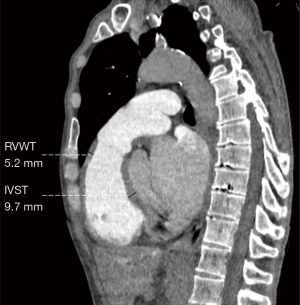
Two radiologists with 5 years of experience in chest imaging measured the cardiovascular metrics on CTPA and reached a consensus, with discussion being used to resolve any differences. On the transversal images of CTPA (Figure 2A), the widest diameter of the main pulmonary artery (MPAd) and the widest diameter of the ascending aorta (AAd) at the same level were measured. The Cobb angle (Figure 2B) was considered to be the rotation angle between the interventricular septum and the line connecting the midpoint of the sternum and the thoracic vertebral spinous process. Meanwhile, a radiologist with 15 years of experience completed multiplane reconstruction and measured the following: the longest longitudinal diameter of the right ventricle (RVld); the longest longitudinal diameter of the left ventricle (LVld); the transverse diameter of the right ventricle (RVtd); the transverse diameter of left ventricle (LVtd) (Figure 3A); the longest longitudinal diameter of the right atrium (RAld) and the transverse diameter of the right atrium (RAtd) (Figure 3A); the longest anteroposterior dimension of the left atrium (LAap) (Figure 3B); the widest left-right dimension of the left atrium (LAlr) (Figure 3B); and the maximum area of the right ventricle (RVa), left ventricle (LVa), right atrium (RAa), and left atrium (LAa) (Figure 3C) on 4-chamber views. The right ventricular free wall thickness (RVWT) and interventricular septal thickness (IVST) at CTPA sagittal position images are also measured and shown in Figure 4. The final measurement result was the average of 3 repeated measurements.
Statistical analysis
Statistical analysis was performed using IBM 27.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test and Shapiro-Wilks test were used to assess the normality of the variables. Normal data are expressed as mean ± standard deviation (SD), and the independent samples t-test was used for comparison in different groups. Nonnormal data are expressed as the median [interquartile range (IQR)], and the Mann-Whitney test was used for multiple comparisons among 3 groups. Count data are expressed as frequency (percentage), and the chi-squared test was used for the comparison of different groups. Univariate and stepwise multivariate binary logistic regression analysis was used to evaluate the independent predictors for CTEPH, and receiver operator characteristic (ROC) curve analysis was performed for cardiovascular parameters and prediction models, with the area under the curve (AUC), sensitivity, and specificity being calculated. The correlations between CTPA cardiovascular metrics, hemodynamics, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were analyzed using Spearman rank correlation analysis. The classification of correlation coefficient (r) was as follows: r≤0.3 indicated no or a very weak correlation, 0.3<r≤0.5 indicated low correlation, 0.5<r≤0.8 indicated moderate correlation, and r>0.8 indicated high correlation. Interobserver consistency was evaluated using intraclass correlation coefficient (ICC), with an ICC greater than 0.8 indicating high consistency. A 2-sided P value of less than 0.05 indicated statistical significance.
Results
Patient characteristics
This study included 180 patients (males n=86; age 55.5±12.0 years old) (Figure 1). Table 1 indicates the demographics, hemodynamics, and clinical characteristics of our study cohort. Patients were placed into groups of mPAP ≤20 mmHg (n=50) and mPAP >20 mmHg (n=130) according to the 2022 ESC/ERS guidelines (1). Among the 50 patients without PH (mPAP =14.7±3.3 mmHg; PVR =1.3 WU), there were 43 patients with chronic thromboembolic pulmonary disease (CTEPD), 4 patients with Takayasu arteritis, 2 patients with fibrosing mediastinitis, and 1 patient with Behcet syndrome. All 130 patients with PH (mPAP >20 mmHg) were diagnosed with CTEPH. Table 1 shows that there were no significant differences in age, gender, or body mass index (BMI) between patients with and without PH (P>0.05). According to the 2015 ESC/ERS guidelines, there were 61 patients with mPAP <25 mmHg and 119 patients with mPAP ≥25 mmHg who were diagnosed as CTEPH. Table 1 also indicates that the age, gender, and BMI between the patients with CTEPH and mPAP >20 mmHg and those with mPAP ≥25 mmHg were comparable (P>0.05).
Table 1
| Characteristics | Total patients (n=180) | mPAP ≤20 mmHg group (n=50) | mPAP > 20 mmHg group (n=130) | mPAP ≥25 mmHg group (n=119) | P valuea | P valueb |
|---|---|---|---|---|---|---|
| Age (years) | 55.5±12.0 | 53.2±12.7 | 56.4±11.6 | 56.3 ±11.2 | 0.101 | 0.916 |
| Gender (male/female) | 86/94 | 26/24 | 60/70 | 54/65 | 0.485 | 0.902 |
| BMI (kg/m2) | 24.5±3.4 | 24.9±3.5 | 24.4±3.3 | 24.3±3.2 | 0.291 | 0.803 |
| NT-proBNP (pg/mL) | 203 [64–876] | 51 [20–84] | 469 [128–1,177] | 518 [170–1,256] | <0.001** | 0.634 |
| 6MWD (m) | 410±116 | 498±89 | 387±112 | 377±111 | <0.001** | 0.529 |
| WHO FC I/II/III/IV | 48/64/53/15 | 34/9/6/1 | 14/55/47/14 | 10/50/45/14 | <0.001** | 0.927 |
| Resting hemodynamics | ||||||
| mPAP (mmHg) | 32.0±14.2 | 14.7±3.3 | 38.6±10.8 | 40.3±10.1 | <0.001** | 0.275 |
| sPAP (mmHg) | 57.0±26.3 | 26.5±6.9 | 68.8±21.0 | 71.7±19.4 | <0.001** | 0.253 |
| dPAP (mmHg) | 18.6±9.0 | 7.8±3.2 | 22.8±6.7 | 23.5±6.5 | <0.001** | 0.345 |
| mRAP (mmHg) | 4 [2–6] | 2 [0.8–4] | 5 [2–7] | 5 [2–7] | <0.001** | 0.774 |
| PAWP (mmHg) | 9.4±2.9 | 9.2±3.0 | 9.5±2.8 | 9.4±2.8 | 0.504 | 0.665 |
| sBP (mmHg) | 131.3±19.8 | 133.2±20.3 | 130.5±19.6 | 130.5±19.8 | 0.434 | 0.985 |
| dBP (mmHg) | 82.9±13.5 | 81.3±12.7 | 83.6±13.8 | 83.9±13.9 | 0.323 | 0.878 |
| PVR (Wood units) | 6.3 [2.1–11.2] | 1.3 [0.7–1.8] | 8.8 [5.8–13.5] | 9.6 [6.3–13.8] | <0.001** | 0.408 |
| CO (L/min) | 3.7±1.3 | 4.6±1.3 | 3.4±1.1 | 3.3±1.1 | <0.001** | 0.721 |
| Cardiac index (L/min/m2) | 2.2±0.7 | 2.6±0.7 | 2.0±0.6 | 1.95±0.6 | <0.001** | 0.751 |
Data are presented as mean ± standard deviation, median [interquartile range], or number (frequency). a, statistical difference between the mPAP >20 mmHg group and the mPAP ≤20 mmHg group; b, statistical difference between the mPAP >20 mmHg group and the mPAP ≥25 mmHg group. **, P<0.001 is considered statistically significant. mPAP, mean pulmonary arterial pressure; BMI, body mass index; NT-proBNP, N-terminal pro-brain natriuretic peptide; 6MWD, 6-minute walking distance; WHO FC, World Health Organization functional class; sPAP, systolic pulmonary arterial pressure; dPAP, diastolic pulmonary arterial pressure; mRAP, mean right atrial pressure; PAWP, pulmonary artery wedge pressure; sBP, systolic blood pressure; dBP, diastolic blood pressure; PVR, pulmonary vascular resistance; CO, cardiac output.
Cardiovascular metrics on CTPA
Table 2 shows the interobserver ICC for cardiovascular metrics from CTPA in all patients, which ranged from 0.818 to 0.977 (P<0.01). Based on the new guidelines, patients with CTEPH had a higher Cobb angle, MPAd, RVtd, RVld, RAtd, RAld, RVa, RAa, RVWT, MPAd/AAd, RVtd/LVtd, RVa/LVa, and RVWT/IVST compared to the patients without PH, as shown in Table 2 (P<0.05). However, Table 3 demonstrates that these cardiovascular metrics including Cobb angle, MPAd, RVtd, RVld, RAtd, RAld, RVa, RAa, RVWT, MPAd/AAd, RVtd/LVtd, RVa/LVa, and RVWT/IVST were comparable between the 2022 and 2015 ESC/ERS criteria (P>0.05).
Table 2
| Variable | Interobserver agreement(ICC, N=180) | Group without PH, mPAP ≤20 mmHg (N=50) | CTEPH group, mPAP >20 mmHg (N=130) |
P value |
|---|---|---|---|---|
| Cobb angle (degree) | 0.970 | 36.7±8.1 | 54.2±13.4 | <0.001** |
| MPAd (mm) | 0.961 | 26.7±3.5 | 35.6±5.4 | <0.001** |
| AAd (mm) | 0.960 | 32.8±5.3 | 32.2±4.8 | 0.447 |
| RVtd (mm) | 0.963 | 35.8±4.0 | 47.7±9.7 | <0.001** |
| RVld (mm) | 0.974 | 67.2±10.4 | 75.0±9.5 | <0.001** |
| RAtd (mm) | 0.912 | 43.3±6.4 | 54.9±11.7 | <0.001** |
| RAld (mm) | 0.904 | 39.4±8.0 | 50.4±11.6 | <0.001** |
| LVtd (mm) | 0.974 | 39.4±6.9 | 36.0±8.0 | 0.009* |
| LVld (mm) | 0.929 | 70.0±8.9 | 67.4±9.7 | 0.096 |
| LAlr (mm) | 0.870 | 55.2±8.4 | 56.3±9.2 | 0.462 |
| LAap (mm) | 0.874 | 39.2±7.3 | 39.0±7.0 | 0.90 |
| RVa (mm2) | 0.957 | 18.7±5.3 | 29.0±9.3 | <0.001** |
| RAa (mm2) | 0.947 | 15.0±4.7 | 24.4±10.5 | <0.001** |
| LVa (mm2) | 0.961 | 24.2±6.5 | 21.6±6.8 | 0.029* |
| LAa (mm2) | 0.928 | 17.5±4.9 | 16.6±5.3 | 0.172 |
| RVWT (mm) | 0.818 | 3.1±1.1 | 5.5±1.6 | <0.001** |
| IVST (mm) | 0.942 | 9.9±2.1 | 9.2±2.1 | 0.056 |
| MPAd/AAd ratio | 0.971 | 0.83±0.15 | 1.13±0.24 | <0.001** |
| RVtd/LVtd ratio | 0.974 | 0.93±0.14 | 1.41±0.50 | <0.001** |
| RVa/LVa ratio | 0.977 | 0.79±0.16 | 1.5±0.80 | <0.001** |
| RVWT/IVST ratio | 0.866 | 0.32±0.11 | 0.63±0.22 | <0.001** |
Continuous variables are expressed as mean value ± standard deviation. *, P<0.05 and **, P<0.001 are considered statistically significant. CTPA, computed tomography pulmonary angiography; mPAP, mean pulmonary arterial pressure; ESC, European Society of Cardiology; ERS, European Respiratory Society; ICC, intraclass correlation coefficient; PH, pulmonary hypertension; CTEPH, chronic thromboembolic pulmonary hypertension; MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta; RVtd, transverse diameter of the right ventricle; RVld, longitudinal diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RAld, longitudinal diameter of the right atrium; LVtd, transverse diameter of the left ventricle; LVld, longitudinal diameter of the left ventricle; LAlr, left-right diameter of the left atrium; LAap, anteroposterior diameter of the left atrium; RVa, area of the right ventricle; RAa, area of the right atrium; LVa, area of the left ventricle; LAa, area of the left atrium; RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
Table 3
| Metric on CTPA | CTEPH group, mPAP >20 mmHg (N=130) | CTEPH group, mPAP ≥25 mmHg (N=119) | P value |
|---|---|---|---|
| Cobb angle (degree) | 54.2±13.4 | 55.6±12.9 | 0.417 |
| MPAd (mm) | 35.6±5.4 | 36.1±5.2 | 0.542 |
| AAd (mm) | 32.2±4.8 | 32.1±4.7 | 0.916 |
| RVtd (mm) | 47.7±9.7 | 48.7±9.3 | 0.405 |
| RVld (mm) | 75.0±9.5 | 75.6±9.2 | 0.581 |
| RAtd (mm) | 54.9±11.7 | 55.7±11.6 | 0.575 |
| RAld (mm) | 50.4±11.6 | 51.0±11.8 | 0.665 |
| LVtd (mm) | 36.0±8.0 | 35.7±8.0 | 0.734 |
| LVld (mm) | 67.4±9.7 | 67.2±9.8 | 0.862 |
| LAlr (mm) | 56.3±9.2 | 56.6±9.1 | 0.789 |
| LAap (mm) | 39.0±7.0 | 38.7±6.8 | 0.765 |
| RVa (mm2) | 29.0±9.3 | 30.0±9.0 | 0.411 |
| RAa (mm2) | 24.4±10.5 | 25.1±10.7 | 0.614 |
| LVa (mm2) | 21.6±6.8 | 21.4±6.8 | 0.762 |
| LAa (mm2) | 16.6±5.3 | 16.5±5.2 | 0.945 |
| RVWT (mm) | 5.5±1.6 | 5.5±1.6 | 0.921 |
| IVST (mm) | 9.2±2.1 | 9.1±2.1 | 0.600 |
| MPAd/AAd ratio | 1.13±0.24 | 1.15±0.24 | 0.652 |
| RVtd/LVtd ratio | 1.41±0.50 | 1.45±0.50 | 0.439 |
| RVa/LVa ratio | 1.5±0.80 | 1.57±0.81 | 0.401 |
| RVWT/IVST ratio | 0.63±0.22 | 0.64±0.23 | 0.608 |
Continuous variables are expressed as mean value ± standard deviation. CTPA, computed tomography pulmonary angiography; mPAP, mean pulmonary arterial pressure; ESC, European Society of Cardiology; ERS, European Respiratory Society; CTEPH, chronic thromboembolic pulmonary hypertension; MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta; RVtd, transverse diameter of the right ventricle; RVld, longitudinal diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RAld, longitudinal diameter of the right atrium; LVtd, transverse diameter of the left ventricle; LVld, longitudinal diameter of the left ventricle; LAlr, left-right diameter of the left atrium; LAap, anteroposterior diameter of the left atrium; RVa, area of the right ventricle; RAa, area of the right atrium; LVa, area of the left ventricle; LAa, area of the left atrium; RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
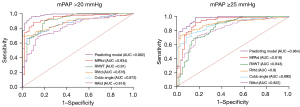
Univariate and forward multivariate binary logistic regression indicated that in both the old and new criteria, MPAd, RVtd, Cobb angle, and RVWT were independent predictors of CTPA for mPAP >20 mmHg (P<0.01) (Table 4). Table 5 shows the ROC analysis of the cardiovascular metrics in the prediction of PH under the old and new criteria. Compared to the other metrics, the MPAd of 30.4 mm exhibited the highest AUC (0.934±0.021) in the diagnosis of mPAP >20 mmHg, with a sensitivity of 0.88 and a specificity of 0.90 (Figure 5).
Table 4
| Variable | Nonstandardized coefficient | Walt value | P value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| β | SE | Lower limit | Upper limit | ||||
| mPAP >20 mmHg | |||||||
| Cobb angle | 0.102 | 0.044 | 5.478 | 0.019* | 1.108 | 1.017 | 1.207 |
| MPAd | 0.240 | 0.095 | 6.354 | 0.012* | 1.271 | 1.055 | 1.532 |
| RVtd | 0.162 | 0.063 | 6.575 | 0.01* | 1.176 | 1.039 | 1.331 |
| RVWT | 1.296 | 0.397 | 10.664 | 0.001** | 3.655 | 1.679 | 7.955 |
| mPAP ≥25 mmHg | |||||||
| Cobb angle | 0.121 | 0.038 | 9.916 | 0.002** | 1.129 | 1.047 | 1.217 |
| MPAd | 0.246 | 0.083 | 8.793 | 0.003** | 1.279 | 1.087 | 1.505 |
| RVtd | 0.185 | 0.057 | 10.415 | 0.001** | 1.203 | 1.075 | 1.345 |
| RVWT | 0.703 | 0.246 | 8.205 | 0.004** | 2.020 | 1.249 | 3.269 |
*, P<0.05 and **, P<0.001 are considered statistically significant. OR, odds ratio; CI confidence interval; SE, standard error; mPAP, mean pulmonary arterial pressure; MPAd, diameter of the main pulmonary artery; RVtd, transverse diameter of the right ventricle; RVWT, the right ventricular free wall thickness.
Table 5
| Cardiovascular metric on CTPA | Cutoff value | AUC | 95% CI | P value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| CTPA metric for mPAP >20 mmHg | |||||||
| Cobb angle (degree) | 48.3 | 0.872±0.026 | 0.822 | 0.922 | <0.001** | 0.67 | 1.00 |
| MPAd (mm) | 30.4 | 0.934±0.021 | 0.892 | 0.976 | <0.001** | 0.88 | 0.90 |
| RVtd (mm) | 43.3 | 0.876±0.025 | 0.827 | 0.925 | <0.001** | 0.69 | 1.00 |
| RAtd (mm) | 49.7 | 0.819±0.033 | 0.755 | 0.883 | <0.001** | 0.69 | 0.86 |
| RVa (mm2) | 24.5 | 0.842±0.031 | 0.781 | 0.904 | <0.001** | 0.72 | 0.86 |
| RAa (mm2) | 15.4 | 0.808±0.034 | 0.742 | 0.875 | <0.001** | 0.83 | 0.66 |
| RVWT (mm) | 3.8 | 0.910±0.025 | 0.861 | 0.959 | <0.001** | 0.90 | 0.80 |
| MPAd/AAd ratio | 0.98 | 0.873±0.027 | 0.821 | 0.925 | <0.001** | 0.76 | 0.84 |
| RVtd/LVtd ratio | 0.99 | 0.860±0.028 | 0.806 | 0.914 | <0.001** | 0.84 | 0.80 |
| RVa/LVa ratio | 0.93 | 0.870±0.026 | 0.819 | 0.920 | <0.001** | 0.79 | 0.84 |
| RVWT/IVST ratio | 0.40 | 0.925±0.020 | 0.886 | 0.964 | <0.001** | 0.90 | 0.80 |
| Prediction model | – | 0.979±0.009 | 0.961 | 0.996 | <0.001** | – | – |
| CTPA metric for mPAP ≥25 mmHg | |||||||
| Cobb angle (degree) | 46.2 | 0.892±0.023 | 0.847 | 0.937 | <0.001** | 0.77 | 0.87 |
| MPAd (mm) | 30.4 | 0.918±0.025 | 0.869 | 0.967 | <0.001** | 0.92 | 0.84 |
| RVtd (mm) | 43.3 | 0.900±0.023 | 0.854 | 0.946 | <0.001** | 0.73 | 0.97 |
| RAtd (mm) | 49.7 | 0.822±0.032 | 0.760 | 0.885 | <0.001** | 0.72 | 0.82 |
| RVa (mm2) | 24.5 | 0.868±0.028 | 0.813 | 0.924 | <0.001** | 0.77 | 0.87 |
| RAa (mm2) | 15.8 | 0.802±0.033 | 0.737 | 0.868 | <0.001** | 0.86 | 0.66 |
| RVWT (mm) | 3.8 | 0.843±0.032 | 0.780 | 0.906 | <0.001** | 0.89 | 0.66 |
| MPAd/AAd ratio | 0.98 | 0.856±0.030 | 0.798 | 0.915 | <0.001** | 0.78 | 0.77 |
| RVtd/LVtd ratio | 0.99 | 0.882±0.025 | 0.833 | 0.930 | <0.001** | 0.87 | 0.85 |
| RVa/LVa ratio | 0.92 | 0.898±0.022 | 0.854 | 0.942 | <0.001** | 0.85 | 0.80 |
| RVWT/IVST ratio | 0.45 | 0.891±0.024 | 0.844 | 0.938 | <0.001** | 0.85 | 0.77 |
| Prediction model | – | 0.964±0.014 | 0.936 | 0.992 | <0.001** | – | – |
Prediction model = Cobb angle + MPAd + RVtd + RVWT. Continuous variables are expressed as mean value ± standard deviation. **, P<0.001 is considered statistically significant. ROC, receiver operator characteristic curve; CTPA, computed tomography pulmonary angiography; ESC, European Society of Cardiology; ERS, European Respiratory Society; AUC, area under ROC curve; CI, confidence interval; mPAP, mean pulmonary arterial pressure; MPAd, diameter of the main pulmonary artery; RVtd, transverse diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RVa, area of the right ventricle; RAa, area of the right atrium; RVWT, right ventricular free wall thickness; AAd, diameter of the ascending aorta; LVtd, transverse diameter of the left ventricle; LVa, area of the left ventricle; IVST, interventricular septal thickness.
Correlation of cardiovascular metrics and hemodynamics with clinical data
Table 6 shows that the Cobb angle (r=0.586 to 0.693), RVtd/LVtd (r=0.583 to 0.596), and RVa/LVa (r=0.629 to 0.652) had a moderate correlation with mPAP, PVR, and NT-proBNP, respectively. Additionally, RVtd (r=0.368 to 0.570), RAtd (r=0.466 to 0.639), RAld (r=0.409 to 0.603), RVa (r=0.319 to 0.581), and RAa (r=0.432 to 0.629) also rspectively exhibited low to moderate correlations with mPAP, mRAP, PVR, and NT-proBNP. MPAd (r=0.482) and MPAd/AAd (r=0.391) had low correlations with mPAP and only weak correlations with PVR, NT-proBNP, and mRAP (r=0.143 to 0.262). There was a low negative correlation (r=−0.45) between CO and Cobb angle, while left ventricular metrics (LVld, LVtd, LVa) showed a low positive correlation (r=0.376 to 0.471) with CO.
Table 6
| Cardiovascular metric on CTPA | mPAP (mmHg) | mRAP (mmHg) | PVR (WU) | PAWP (mmHg) | CO (L/min) | NT-proBNP (pg/mL) |
|---|---|---|---|---|---|---|
| Cobb angle (degree) | 0.586** | 0.328** | 0.613** | −0.114 | −0.450** | 0.693** |
| MPAd (mm) | 0.482** | 0.218* | 0.262** | 0.037 | −0.042 | 0.230 ** |
| AAd (mm) | −0.074 | −0.005 | −0.058 | 0.041 | −0.080 | −0.021 |
| RVtd (mm) | 0.518** | 0.368** | 0.428** | −0.067 | −0.191* | 0.570** |
| RVld (mm) | 0.182** | 0.174* | 0.080 | 0.064 | 0.046 | 0.223** |
| RAtd (mm) | 0.558** | 0.466** | 0.478** | 0.011 | −0.258** | 0.639** |
| RAld (mm) | 0.467** | 0.409** | 0.425** | 0.030 | −0.289** | 0.603** |
| LVtd (mm) | −0.333** | −0.032 | −0.43** | 0.194* | 0.376** | −0.307** |
| LVld (mm) | −0.312** | −0.068 | −0.444** | 0.088 | 0.471** | −0.346** |
| LAlr (mm) | 0.151 | 0.186* | 0.090 | 0.091 | 0.019 | 0.082 |
| LAap (mm) | −0.079 | 0.111 | −0.211* | 0.115 | 0.257* | −0.146 |
| RVa (mm2) | 0.487** | 0.319** | 0.410** | −0.068 | −0.190* | 0.581** |
| RAa (mm2) | 0.495** | 0.432** | 0.442** | −0.002 | 0.270** | 0.629** |
| LVa (mm2) | −0.335** | −0.035 | −0.438** | 0.138 | 0.430** | −0.294** |
| LAa (mm2) | −0.023 | −0.118 | −0.164* | 0.209* | 0.248** | −0.115 |
| RVWT (mm) | 0.129 | 0.041 | −0.013 | 0.148 | 0.119 | −0.014 |
| IVST (mm) | −0.177* | −0.081 | −0.259** | 0.159 | 0.218* | −0.257** |
| MPAd/AAd ratio | 0.391** | 0.143* | 0.222* | 0.035 | 0.042 | 0.200* |
| RVtd/LVtd ratio | 0.583** | 0.248* | 0.593** | −0.174 | −0.382** | 0.596** |
| RVa/LVa ratio | 0.629** | 0.265** | 0.642** | −0.146 | −0.442** | 0.652** |
| RVWT/IVST ratio | 0.266** | 0.121 | 0.197* | −0.024 | −0.069 | 0.205* |
*, P<0.05 and **, P<0.001 are considered statistically significant. CTPA, computed tomography pulmonary angiography; NT-proBNP, N-terminal pro-brain natriuretic peptide; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PVR, pulmonary vascular resistance; WU, Wood units; PAWP, pulmonary artery wedge pressure; CO, cardiac output; MPAd, diameter of the main pulmonary artery; AAd, diameter of the ascending aorta; RVtd, transverse diameter of the right ventricle; RVld, longitudinal diameter of the right ventricle; RAtd, transverse diameter of the right atrium; RAld, longitudinal diameter of the right atrium; LVtd, transverse diameter of the vleft ventricle; LVld, longitudinal diameter of the left ventricle; LAlr, left-right diameter of the left atrium; LAap, anteroposterior diameter of the left atrium; RVa, area of the right ventricle; RAa, area of the right atrium; LVa, area of the left ventricle; LAa, area of the left atrium; RVWT, right ventricular free wall thickness; IVST, interventricular septal thickness.
Discussion
Due to the changes in the mPAP criteria for diagnosing PH, we comprehensively compared the cardiovascular metrics from CTPA in CTEPH between the 2022 ESC/ERS guidelines (mPAP >20 mmHg) (1) and the 2015 ESC/ERS guidelines (mPAP ≥25 mmHg) (2). To our knowledge, this is the first study to analyze the difference in cardiovascular metrics on CTPA under the old and updated criteria. Our study produced several major findings: (I) there was no statistically significant difference in cardiovascular metrics for the diagnosis of PH between new and old guidelines; (II) MPAd, RVtd, Cobb angle, and RVWT on CTPA were independent predictors for mPAP >20 mmHg in patients with CTEPH; (III) compared to the other metrics, an MPAd of 30.4 mm exhibited the highest AUC, with a sensitivity of 0.88 and specificity of 0.90; (IV) cardiovascular metrics, especially metrics from the 4-chamber view of CTPA images moderately correlated with mPAP.
CTPA is a promising choice as the primary imaging method for suspected CTEPH due to its speed, wide availability, excellent spatial and temporal resolution, and ability to directly visualize chronic pulmonary embolism (16,17). Its high sensitivity and specificity for CTEPH allow for early diagnosis and help avoid advanced disease stages in patients with CTEPH (16). According to the 2022 ERS/ERS guidelines for the diagnosis of PH, cardiovascular metrics from CTPA, including Cobb angle, MPAd, RVtd, RVld, RAtd, RAld, RVa, RAa, RVWT, MPAd/AAd, RVtd/LVtd, RVa/LVa, and RVWT/IVST were significantly higher in patients with CTEPH than in patients without PH. These results are similar to those reported by Charters et al. (13) and Liu et al. (21), indicating that cardiovascular metrics on CTPA can predict mPAP >20 mmHg. Cardiovascular metrics from CTPA have been applied to assess PH (1,2,12,18). For instance, a cutoff value of 29 mm for MPAd has been used as an indicator of mPAP ≥25 mmHg. Corson et al. (22) reported an MPAd/AAd >1 as being an imaging marker of PH. Recently, a meta-analysis indicated that CT measurement MPAd/AAd ratio ≥1 has a combined sensitivity of 0.652 [95% confidence interval (CI): 0.579–0.719] and a combined specificity of 0.830 (95% CI: 0.796–0.880) in predicting mPAP ≥25 mmHg (12). In our study, according to the 2022 ERS/ERS guidelines, the sensitivity and specificity of a 30.4 mm cutoff value for MPAd in predicting mPAP >20 mmHg, respectively, was 0.88 and 0.90, whereas the sensitivity and specificity of a 0.98 cutoff value for MPAd/AAd in predicting mPAP >20 mmHg, respectively, was 0.76 and 0.84 in Table 5. Notably, both RVWT and RVWT/IVST ratio had a sensitivity of 0.90 and specificity of 0.80 in diagnosing mPAP >20 mmHg. Similarly, Swift et al. proposed that an MPAd of 30 mm represents a compromise threshold for identifying patients with mPAP >20 mmHg (5). In our study, Table 5 shows the cutoff value of MPAd/AAd and other parameters indicating RV enlargement under the new guidelines were also similar to those of previous studies (12,23,24).
In current study, there was no statistically significant differences in cardiovascular metrics between patients with mPAP >20 mmHg and mPAP ≥25 mmHg. The lack of differences in the cardiovascular parameters of CTPA between the old and new criteria for PH may be attributed to the following: (I) the difference of 4 mmHg in mPAP may have a limited impact on the morphological changes in the pulmonary artery and right heart; (II) the lower mPAP criterion may not fully capture the clinical condition or reflect the underlying pathological process itself (19); (III) the few cases with mPAP between 21 and 24 mmHg might have limited the significant differences in cardiovascular parameters between the old and new diagnostic criteria; (IV) the accuracy of cutoff values for cardiovascular parameters may be influenced by various factors, such as patient selection representing different subtypes of PH. Further investigations with larger cohorts are needed to confirm these findings.
In addition, our study demonstrated that Cobb angle, MPAd, RVtd, and RVWT on CTPA were independent predictors of PH for patients with suspected CETPH regardless of whether the new or old diagnostic criteria were used. Previous studies have focused on the impact of right ventricular changes on PH (14,25,26), while the right atrium in CTPA has been largely overlooked. Our study found that the RAtd in the 4-chamber view moderately correlated with mPAP. This indicates that RAtd may reflect mPAP and aid in PH evaluation (4). The Cobb angle also showed a moderate correlation with mPAP and PVR, and its cutoff value of 48.3° for predicting PH with mPAP >20 mmHg had high specificity and low sensitivity.
Limitations
There are some limitations in this study. First, we employed a single-center retrospective study design. As there were few cases of other subtypes of PH cases, they were excluded, with only patients with CTEPH being analyzed; this inevitably limits the generalizability of these findings to other types of PH. Therefore, future studies will include cases with precapillary and postcapillary PH. Second, only mPAP was used as the grouping criteria, and thus the alterations of cardiovascular metrics on CTPA under the different PVRs (2 and 3 WU) remain unknown. Third, RHC is not a routine procedure for healthy individuals, so only a small number of patients with normal and mild PH were included. Whether cardiovascular metrics in patients with mPAP ranging from 21 to 24 mmHg are different from those of patients with mPAP ≤20 mmHg is unclear, and the optimal cutoff values of cardiovascular metrics on CTPA in PH patients need to be further investigated.
Conclusions
Cardiovascular metrics on CTPA were similar between the updated and old PH guidelines. MPAd, Cobb angle, RVtd, and RVWT on CTPA were independent predictors of mPAP >20 mmHg. An MPAd of 30.4 mm represents a compromise threshold for identifying mPAP >20 mmHg in patients with CTEPH.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-250/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-250/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional ethics board of China-Japan Friendship Hospital (No. 2022-KY-048). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz SESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618-731. Erratum in: Eur Heart J 2023;44:1312.
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper MESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Klok FA, Barco S, Konstantinides SV, Dartevelle P, Fadel E, Jenkins D, Kim NH, Madani M, Matsubara H, Mayer E, Pepke-Zaba J, Delcroix M, Lang IM. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH Registry. Eur Respir J 2018;52:1801687. [Crossref] [PubMed]
- Tsukada J, Yamada Y, Kawakami T, Matsumoto S, Inoue M, Nakatsuka S, Okada M, Fukuda K, Jinzaki M. Treatment effect prediction using CT after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Radiol 2021;31:5524-32. [Crossref] [PubMed]
- Swift AJ, Dwivedi K, Johns C, Garg P, Chin M, Currie BJ, Rothman AM, Capener D, Shahin Y, Elliot CA, Charalampopolous T, Sabroe I, Rajaram S, Hill C, Wild JM, Condliffe R, Kiely DG. Diagnostic accuracy of CT pulmonary angiography in suspected pulmonary hypertension. Eur Radiol 2020;30:4918-29. [Crossref] [PubMed]
- Liu M, Ma Z, Guo X, Chen X, Yang Y, Wang C. Cardiovascular parameters of computed tomographic pulmonary angiography to assess pulmonary vascular resistance in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol 2013;164:295-300. [Crossref] [PubMed]
- Wittenberg R, van Vliet JW, Ghaye B, Peters JF, Schaefer-Prokop CM, Coche E. Comparison of automated 4-chamber cardiac views versus axial views for measuring right ventricular enlargement in patients with suspected pulmonary embolism. Eur J Radiol 2012;81:218-22. [Crossref] [PubMed]
- Lu MT, Demehri S, Cai T, Parast L, Hunsaker AR, Goldhaber SZ, Rybicki FJ. Axial and reformatted four-chamber right ventricle-to-left ventricle diameter ratios on pulmonary CT angiography as predictors of death after acute pulmonary embolism. AJR Am J Roentgenol 2012;198:1353-60. [Crossref] [PubMed]
- Liu M, Ma Z, Guo X, Zhang H, Yang Y, Wang C. Computed tomographic pulmonary angiography in the assessment of severity of chronic thromboembolic pulmonary hypertension and right ventricular dysfunction. Eur J Radiol 2011;80:e462-9. [Crossref] [PubMed]
- Stein PD, Matta F, Yaekoub AY, Goodman LR, Sostman HD, Weg JG, Hales CA, Hull RD, Leeper KV Jr, Beemath A, Saeed IM, Woodard PK. Reconstructed 4-chamber views compared with axial imaging for assessment of right ventricular enlargement on CT pulmonary angiograms. J Thromb Thrombolysis 2009;28:342-7. [Crossref] [PubMed]
- Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021;57:2002828. [Crossref] [PubMed]
- Chen R, Liao H, Deng Z, He Z, Zheng Z, Lu J, Jiang M, Wu X, Guo W, Huang Z, Chen H, Hong C, Zhong N. Efficacy of computed tomography in diagnosing pulmonary hypertension: A systematic review and meta-analysis. Front Cardiovasc Med 2022;9:966257. [Crossref] [PubMed]
- Charters PFP, Rossdale J, Brown W, Burnett TA, Komber HMEI, Thompson C, Robinson G, MacKenzie Ross R, Suntharalingam J, Rodrigues JCL. Diagnostic accuracy of an automated artificial intelligence derived right ventricular to left ventricular diameter ratio tool on CT pulmonary angiography to predict pulmonary hypertension at right heart catheterisation. Clin Radiol 2022;77:e500-8. [Crossref] [PubMed]
- Freed BH, Collins JD, François CJ, Barker AJ, Cuttica MJ, Chesler NC, Markl M, Shah SJ MR. JACC Cardiovasc Imaging 2016;9:715-32. [Crossref] [PubMed]
- François CJ, Schiebler ML. Imaging of Pulmonary Hypertension. Radiol Clin North Am 2016;54:1133-49. [Crossref] [PubMed]
- Lambert L, Michalek P, Burgetova A. The diagnostic performance of CT pulmonary angiography in the detection of chronic thromboembolic pulmonary hypertension-systematic review and meta-analysis. Eur Radiol 2022;32:7927-35. [Crossref] [PubMed]
- McInnis M. Imaging Advances in Chronic Thromboembolic Pulmonary Hypertension. Semin Roentgenol 2022;57:324-34. [Crossref] [PubMed]
- Maschke SK, Werncke T, Dewald CLA, Becker LS, Meine TC, Olsson KM, Hoeper MM, Wacker FK, Meyer BC, Hinrichs JB. Depiction of mosaic perfusion in chronic thromboembolic pulmonary hypertension (CTEPH) on C-arm computed tomography compared to computed tomography pulmonary angiogram (CTPA). Sci Rep 2021;11:20042. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Maron BA, Kovacs G, Vaidya A, Bhatt DL, Nishimura RA, Mak S, Guazzi M, Tedford RJ. Cardiopulmonary Hemodynamics in Pulmonary Hypertension and Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:2671-81. [Crossref] [PubMed]
- Liu M, Ma ZH, Guo XJ, Wang SK, Chen XY, Yang YH, Wang C. A septal angle measured on computed tomographic pulmonary angiography can noninvasively estimate pulmonary vascular resistance in patients with chronic thromboembolic pulmonary hypertension. J Thorac Imaging 2012;27:325-30. [Crossref] [PubMed]
- Corson N, Armato SG 3rd, Labby ZE, Straus C, Starkey A, Gomberg-Maitland M. CT-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol 2014;21:523-30. [Crossref] [PubMed]
- Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014;145:824-32. [Crossref] [PubMed]
- Rajaram S, Swift AJ, Capener D, Elliot CA, Condliffe R, Davies C, Hill C, Hurdman J, Kidling R, Akil M, Wild JM, Kiely DG. Comparison of the diagnostic utility of cardiac magnetic resonance imaging, computed tomography, and echocardiography in assessment of suspected pulmonary arterial hypertension in patients with connective tissue disease. J Rheumatol 2012;39:1265-74. [Crossref] [PubMed]
- Beyar R, Dong SJ, Smith ER, Belenkie I, Tyberg JV. Ventricular interaction and septal deformation: a model compared with experimental data. Am J Physiol 1993;265:H2044-56. [Crossref] [PubMed]
- Tsujimoto Y, Kumasawa J, Shimizu S, Nakano Y, Kataoka Y, Tsujimoto H, Kono M, Okabayashi S, Imura H, Mizuta T. Doppler trans-thoracic echocardiography for detection of pulmonary hypertension in adults. Cochrane Database Syst Rev 2022;5:CD012809. [Crossref] [PubMed]


