
Exceptional response to immunotherapy monotherapy in a patient with an unfavorable subset of cancer of unknown primary
Introduction
Cancer of unknown primary (CUP) is a heterogeneous malignant condition characterized by the absence of a primary site of origin following general diagnostic evaluation (1). It accounts for approximately 3–5% of all malignancies and exhibits clinical features, including early metastasis, hyperinvasiveness, and an unpredictable metastatic pattern (1). In addition, about 3% of melanomas, distinguished by the absence of an identifiable primary site, are commonly recognized as melanoma of unknown primary (MUP). This subtype, contrasting with the classical melanoma with known primary (MKP), remains undefined in biological terms. Recent research indicates that MUP patients receiving immunotherapy could present a superior prognosis compared to MKP patients, possibly related to the heightened immunogenicity in MUP patients as evidenced by the immune-mediated regression of the primary site (2).
Standard treatment options for CUP are lacking, and systemic empirical chemotherapy is the most commonly employed approach (3). Recently, immunotherapy has emerged as a promising avenue of research for CUP, providing a potential alternative treatment strategy for patients. Chebly et al. (4) reported that individual gene mutations in CUP patients with chromosomal instability (CIN) are associated with immune escape and resistance to immune checkpoint inhibitors (ICI). Conversely, CIN is infrequently identified in patients with CUP, which may support the utilization of ICI in this patient population. Nevertheless, the complex interplay among point mutations, CIN, and the immune system necessitates further investigation to broaden our comprehension of ICI application within CUP patients, with the potential to enhance therapeutic efficacy. In the present study, we report the case of a patient with CUP involving the liver and splenic hilum who achieved a favorable treatment response and significant survival benefit after being treated with a programmed death-1 (PD-1) ICI monotherapy.
Case presentation
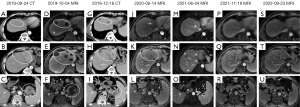
On 23 September 2019, a 65-year-old male patient was admitted to the Jiangyin Hospital Affiliated to Nantong University because of abdominal pain that had persisted for 1 week. During hospitalization, the patient underwent a series of tumor histopathology examinations, tumor marker assessments (Figure 1), and imaging assessments (Figure 2). The specific diagnostic and treatment process was as follows. The patient had undergone radical rectal cancer surgery on 16 May 2011, which revealed mucinous adenocarcinoma of the sigmoid colon (Figure 1A). After admission, an abdominal and pelvic enhanced computed tomography (CT) scan (Figure 2A-2C) showed multiple intrahepatic masses (with the largest measuring 2.5 cm × 2.3 cm) and a mass in the splenic hilum area (measuring 5.6 cm × 5.4 cm), raising suspicions of metastases. Although gastrointestinal endoscopy did not detect tumor recurrence, metastases were highly suspected to have originated from colon cancer recurrence. Starting from 27 September 2019, the patient developed a fever without chills, with an initial body temperature of 38.4 ℃. Despite receiving appropriate anti-inflammatory and antipyretic treatment, the patient’s febrile symptoms persisted, with the temperature reaching a maximum of 39.9 ℃. Repeated routine blood tests and blood bacteriology tests returned negative results. Among the elevated tumor markers, serum carbohydrate antigen 125 (CA125) and carbohydrate antigen 199 (CA199) were observed, whereas all other tumor markers remained within the normal range. On 4 October 2019, a magnetic resonance (MR) scan (Figure 2D-2F) showed that the intrahepatic masses had increased in size, the largest of which measured 3.5 cm × 3.4 cm. The short-term intrahepatic tumor size increase was significant, occurring over a period of only 10 days, and was accompanied by a rapid deterioration in performance status (PS; rapidly rising from 2 to 4).
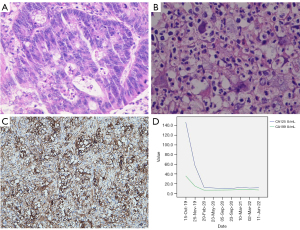
A percutaneous liver tumor biopsy was performed, and the pathology report revealed infiltration of acute and chronic inflammatory cells, without any definitive tumor lesions identified. Despite this finding, the patient expressed a strong desire for treatment, so we consulted with a general surgeon and performed a “laparoscopic partial hepatectomy” on 17 October 2019 to remove a superficial mass located in the left lateral lobe of the liver. The resected mass was then subjected to pathological and immunohistochemical examination. However, the pathologist’s diagnosis indicated a tendency toward poorly differentiated adenocarcinoma (Figure 1B) but failed to identify the tissue source. Given the uncertain diagnosis, we decided to transfer the specimens to the department of pathology at Fudan University Shanghai Cancer Center for further consultation. We also sent the liver tumor specimens to Shanghai 3DMed Diagnostics Medical Laboratory Co., Ltd. (Shanghai, China) for next-generation sequencing (NGS) and immune checkpoint detection. The pathology department of Fudan University Shanghai Cancer Center provided a consultation opinion as follows: immunohistochemistry (IHC) of colon adenocarcinoma showed hMSH6(−), hMSH2(+), hMLH-1(+), PMS2(+), CK20(+), CDX2(+), CK7(−), and EBER(−). IHC of liver poorly differentiated carcinoma showed AE1/AE3(+), CK19(+), weak EMA(+), weak CK7(+), weak CK8(+), CDX2(−), CK20(−), TTF-1(−), Hep-1(−), GPC-3(−), Arg-1(−), CD34(−), p504s(−), LCA(−), CD68(−), Vimentin(+), CD21(−), CD30(−), and EBER(−). The morphology and IHC of the colon adenocarcinoma and liver poorly differentiated carcinoma in this case were different, and there was no definite evidence to suggest that they originated from the same source. Therefore, the primary site could not be identified, and the patient was ultimately diagnosed with CUP.
The NGS analysis revealed that the tumor mutational burden (TMB) was 4.47 Muts/Mb. Additionally, the immune checkpoint detection demonstrated that the tumor proportion score (TPS) of programmed death ligand 1 (PD-L1) was 70% (Figure 1C). No mutations were detected in the genes (ALK, BRAF, BRCA1, BRCA2, CD274, EGFR, ERBB2, FGFR2, FGFR3, KIT, KRAS, MET, NRAS, NTRK1, NTRK2, NTRK3, PDGFRA, PIK3CA, RET, ROS1) analyzed by NGS. Therefore, PD-1 ICI therapy was recommended. From 4 November 2019, the patient received regular doses of camrelizumab (Hengrui Pharmaceutical Co., Ltd., Suzhou, China) at 200 mg, IVD, Q3W. After 2 cycles of Camrelizumab monotherapy, the patient’s febrile symptoms disappeared, and his PS score improved from 4 to 1. No significant adverse reactions were observed during the immunotherapy. The metastatic tumors continued to shrink (Figure 2G-2R), and the elevated tumor markers gradually decreased to normal levels (Figure 1D). A CT scan conducted on 16 December 2019 showed that the largest intrahepatic tumor was about 2 cm × 2 cm, and the tumor in the splenic hilum was 3.9 cm × 3.4 cm (Figure 2G-2I). An MR conducted on 14 June 2020 revealed that the largest intrahepatic tumor was about 1.3 cm × 1.1 cm, and the tumor in the splenic hilum had completely disappeared (Figure 2J-2L). An MR conducted on 18 November 2021, demonstrated that the multiple tumors in the liver had completely disappeared, and the splenic hilum remained tumor-free (Figure 2P-2R). As a result, a complete response was observed, and the immunotherapy was discontinued. To date, the patient remains tumor-free (Figure 2S-2U), with a PS score of 0 and a positive mood. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CUP is an aggressive malignancy with a poor prognosis, as evidenced by reported median survival times ranging from 6 to 9 months (3). Culine et al. (5) categorized CUP patients into favorable and unfavorable subsets according to their PS and lactate dehydrogenase (LDH) levels. The median survival periods for these subsets were 11.7 and 3.9 months, respectively. The majority of CUP patients (80–85%) fall into the unfavorable subset. The case of CUP involving the liver reported in the current study was categorized under the ‘unfavorable subset’ (3), with a notably short median survival time of only 2 months (6). Poor PS is also an independent risk factor for a worse prognosis in CUP (3). Our case presented with a high fever and a significant increase in tumor size accompanied by rapid physical deterioration, indicating an extremely aggressive and rapidly progressive form of CUP that belonged to the unfavorable subset. The favorable subset (15–20%) encompasses peritoneal adenocarcinomatosis of a serous papillary subtype, neuroendocrine carcinomas of unknown primary, isolated axillary lymph node metastasis in females, single metastatic lesion of unknown primary, squamous cell carcinoma involving non-supraclavicular cervical lymph nodes, and males with bone metastases and prostate-specific antigen expression. It also includes colorectal, lung, and renal CUP (7).
Due to the lack of standard treatment for CUP, chemotherapy remains the main treatment strategy. Recent studies have suggested that both empirical and organ-specific chemotherapy offer limited survival benefits for CUP patients (8,9). Despite this, these comparative studies between site-specific treatment and empirical chemotherapy bear significant shortcomings. They encompass challenges inherent to patient acquisition, specifically the overrepresentation of treatment-resistant tumor types and extended recruitment periods. Further complexities are found in the limitations of the study design, notably the difficulties posed by observational trials and trials with suboptimal design. Additionally, a lack of uniformity exists among CUP classifiers, as evidenced by the disparate findings from epigenetic and transcriptomic trials. Moreover, there is a lack of standardization in the therapeutic approaches, rendering comparison a challenge. To address these issues, the implementation of 2 comprehensive clinical trial designs—visionary and pragmatic—may offer potential solutions. These could significantly enhance the caliber of CUP research, thereby improving the prognosis for a substantial patient population.
In the contemporary epoch of targeted therapeutics, the precise histopathological and molecular classification of tumors is of paramount importance to deliver optimal individualized treatment strategies. Classification rooted in epigenetic changes fulfills this role. Indeed, a characteristic of cancer cells lies in their extensive overall reduction of DNA methylation, characterized by a significant 20–60% reduction in 5-methylcytosine. Concurrently, these cells acquire distinctive patterns of hypermethylation at CpG islands within specific promoters. These alterations, which may be either reversible or irreversible, fundamentally modify gene function and subsequently play a pivotal role in the progression of malignancy (10). Recently, personalized treatment strategies based on NGS have been proposed. In a phase II clinical trial of NGS-based site-specific therapy in 97 patients with CUP, the 1-year survival rate was 53%, with a median survival of 13.7 months, suggesting the potential clinical application value of NGS-based molecularly targeted therapy (11). However, another study reported that targeted therapy for CUP patients based on NGS analysis exhibited a 30% incidence of targetable genomic alterations amongst a cohort of 150 CUP patients. Further, it was observed that 10% of these patients were administered targeted therapy. The outcomes, however, demonstrated a substantial range of variability, evidenced by the time-to-treatment failure spanning between 1 to 14 months (12). These findings indicate that targeted therapy based on NGS analysis is still in its early stages.
In recent years, immunotherapy using PD-1/PD-L1 inhibitors has gained considerable attention in the treatment of CUPs. Mei et al. (13) reported a significantly prolonged survival with the administration of pembrolizumab in combination with chemotherapy and local radiotherapy in a CUP patient with high expression of PD-L1 (TPS of PD-L1: 80%) and high TMB of 16.7 Muts/Mb. Similarly, Singh et al. (14) reported a favorable therapeutic response to pembrolizumab in a patient with heart-involved CUP and overexpression of PD-L1 (TPS of PD-L1: 100%). These studies suggested that immunotherapy may emerge as an important treatment option for CUPs. The PD-1/PD-L1 signaling pathway is a critical immune checkpoint pathway that facilitates tumor immune evasion and promotes tumor growth. Targeting PD-1 or PD-L1 with specific antibodies can boost T cell responses and induce antitumor effects (9). Camrelizumab is a PD-1 inhibitor; however, its use in the treatment of CUP has not been reported to date. The administration of camrelizumab in our case was off-label and posed a challenging experimental therapy.
The most widely recognized biomarker for predicting response to PD-1/PD-L1 inhibitors is the expression of PD-L1 in tumor and tumor-infiltrating immune cells. A recent meta-analysis of 41 clinical trials involving 6,664 patients with various advanced solid tumors showed that the objective response rate (ORR) of PD-1/PD-L1 inhibitors was significantly higher in PD-L1 positive patients than in PD-L1 negative patients [odds ratio (OR), 2.26; 95% confidence interval (CI): 1.85–2.75; P<0.001], indicating that positive PD-L1 expression can predict the response to immunotherapy (15). TMB is another emerging biomarker, and a significant positive correlation between TMB and ORR has been observed in various solid cancers (16), suggesting that TMB is also helpful in predicting the efficacy of immunotherapy.
Camrelizumab was recommended because of the high expression of PD-L1 on tumor cells in our case. After immunotherapy, the tumor lesions continued to shrink until complete disappearance, and the PS score improved from 4 to normal (PS =0). Moreover, no tumor recurrence was observed during follow-up after discontinuation of camrelizumab. To our knowledge, this was the first report of a favorable and durable response to PD-1 inhibitor monotherapy in a CUP patient with liver and splenic hilum involvement.
This case provides evidence for the use of immunotherapy in CUP tumors with predictable biomarkers. Notably, 28% of CUP patients exhibit at least 1 predictive biomarker for ICI. These include at least 5% of cancer cells expressing PD-L1 in 22.5% of patients, high microsatellite instability in 1.8% of patients, and a TMB of ≥17 Muts/Mb in 11.8% of patients. Nevertheless, these biomarkers have yet to undergo specific validation in the context of CUP (17). The case highlighted in the current study suggests that CUP patients with high PD-L1 expression are likely to derive benefit from immunotherapy. Moreover, a discernible trend towards improved outcomes with ICI treatment is observed in CUP patients demonstrating a TMB exceeding 10 Muts/Mb (17). Interestingly, despite our case being a TMB-low patient (4.47 Muts/Mb), immunotherapy still exhibited significant efficacy. This suggests that the high expression of PD-L1 in this patient contributed more to the positive response to immunotherapy.
However, in many countries, ICI therapy cannot be used due to regulatory reasons. China also lacks clear legislation for off-label drug usage. Nevertheless, this patient’s disease progressed rapidly, the patient had a high likelihood of dying in the short term, and the survival time may not have exceeded 3 months. The patient’s NGS results showed a high TPS (70%), indicating a high likelihood of benefiting from ICI therapy. Therefore, after fully weighing the benefits and risks of ICI treatment, we recommended ICI therapy for the patient. Nevertheless, before applying ICI, we informed the patient fully and in detail about the potential adverse reactions, complications, or other unexpected situations related to immunotherapy. The patient expressed understanding of the potential risks of immunotherapy and signed an informed consent form for off-label use of ICI therapy. Additionally, there is still controversy regarding the impact of PS on ICI therapy. The 2021 edition of the Chinese Society of Clinical Oncology (CSCO) “Guidelines for the Management of Immune Checkpoint Inhibitor-Related Toxicities” suggested that caution should be exercised in using ICIs for patients with PS scores ≥2 (Class 2A, Level II recommendation). However, a recent meta-analysis published online in JAMA Network Open (18) concluded that compared to control therapies, ICI significantly improves survival rates in many cancer types, regardless of patients’ gender, age, or Eastern Cooperative Oncology Group (ECOG) PS scores. The authors proposed that these results may encourage more patients to receive ICIs and that immunotherapy should not be refused based on gender, age, or PS scores. In this case, the patient had a strong desire to survive, an eagerness to undergo treatment, and he was willing to accept the risks associated with ICI therapy. Therefore, we performed ICI therapy on the patient, and fortunately, the patient achieved a favorable treatment outcome.
Overall, this study describes a case of CUP with liver and splenic hilar involvement as the predominant manifestation. Although achieving a cure for this type of tumor is a rare event, the patient in this study obtained a significant survival benefit with camrelizumab monotherapy alone. This indicates that CUP patients with a biomarker for immunotherapy, such as high expression of PD-L1, may derive benefit from immunotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-284/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet 2012;379:1428-35. [Crossref] [PubMed]
- Boussios S, Rassy E, Samartzis E, Moschetta M, Sheriff M, Pérez-Fidalgo JA, Pavlidis N. Melanoma of unknown primary: New perspectives for an old story. Crit Rev Oncol Hematol 2021;158:103208. [Crossref] [PubMed]
- Losa F, Soler G, Casado A, Estival A, Fernández I, Giménez S, Longo F, Pazo-Cid R, Salgado J, Seguí MÁ. SEOM clinical guideline on unknown primary cancer (2017). Clin Transl Oncol 2018;20:89-96. [Crossref] [PubMed]
- Chebly A, Yammine T, Boussios S, Pavlidis N, Rassy E. Chromosomal instability in cancers of unknown primary. Eur J Cancer 2022;172:323-5. [Crossref] [PubMed]
- Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, Lortholary A, Merrouche Y, Laplanche A, Fizazi KFrench Study Group on Carcinomas of Unknown Primary. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol 2002;20:4679-83. [Crossref] [PubMed]
- Riihimäki M, Thomsen H, Hemminki A, Sundquist K, Hemminki K. Comparison of survival of patients with metastases from known versus unknown primaries: survival in metastatic cancer. BMC Cancer 2013;13:36. [Crossref] [PubMed]
- Rassy E, Parent P, Lefort F, Boussios S, Baciarello G, Pavlidis N. New rising entities in cancer of unknown primary: Is there a real therapeutic benefit? Crit Rev Oncol Hematol 2020;147:102882. [Crossref] [PubMed]
- Rassy E, Bakouny Z, Choueiri TK, Van Allen EM, Fizazi K, Greco FA, Pavlidis N. The role of site-specific therapy for cancers of unknown of primary: A meta-analysis. Eur J Cancer 2020;127:118-22. [Crossref] [PubMed]
- Rassy E, Labaki C, Chebel R, Boussios S, Smith-Gagen J, Greco FA, Pavlidis N. Systematic review of the CUP trials characteristics and perspectives for next-generation studies. Cancer Treat Rev 2022;107:102407. [Crossref] [PubMed]
- Moran S, Martinez-Cardús A, Boussios S, Esteller M. Precision medicine based on epigenomics: the paradigm of carcinoma of unknown primary. Nat Rev Clin Oncol 2017;14:682-94. [Crossref] [PubMed]
- Hayashi H, Takiguchi Y, Minami H, Akiyoshi K, Segawa Y, Ueda H, Iwamoto Y, Kondoh C, Matsumoto K, Takahashi S, Yasui H, Sawa T, Onozawa Y, Chiba Y, Togashi Y, Fujita Y, Sakai K, Tomida S, Nishio K, Nakagawa K. Site-Specific and Targeted Therapy Based on Molecular Profiling by Next-Generation Sequencing for Cancer of Unknown Primary Site: A Nonrandomized Phase 2 Clinical Trial. JAMA Oncol 2020;6:1931-8. [Crossref] [PubMed]
- Varghese AM, Arora A, Capanu M, Camacho N, Won HH, Zehir A, Gao J, Chakravarty D, Schultz N, Klimstra DS, Ladanyi M, Hyman DM, Solit DB, Berger MF, Saltz LB. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann Oncol 2017;28:3015-21. [Crossref] [PubMed]
- Mei J, Wang H, Fan H, Ding J, Xu J. Case Report: Successful Immunotherapy Improved the Prognosis of the Unfavorable Subset of Cancer of Unknown Primary. Front Immunol 2022;13:900119. [Crossref] [PubMed]
- Singh A, Singh D, Boyes S, Henry E. Treatment of Metastatic Carcinoma of an Unknown Primary to the Heart with Checkpoint Inhibitor Therapy. Oncol Res Treat 2021;44:190-5. [Crossref] [PubMed]
- Khunger M, Hernandez AV, Pasupuleti V, Rakshit S, Pennell NA, Stevenson J, Mukhopadhyay S, Schalper K, Velcheti V. Programmed Cell Death 1 (PD-1) Ligand (PD-L1) Expression in Solid Tumors As a Predictive Biomarker of Benefit From PD-1/PD-L1 Axis Inhibitors: A Systematic Review and Meta-Analysis. JCO Precis Oncol 2017;1:1-15. [Crossref] [PubMed]
- Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44-56. [Crossref] [PubMed]
- Rassy E, Boussios S, Pavlidis N. Genomic correlates of response and resistance to immune checkpoint inhibitors in carcinomas of unknown primary. Eur J Clin Invest 2021;51:e13583. [Crossref] [PubMed]
- Yang F, Markovic SN, Molina JR, Halfdanarson TR, Pagliaro LC, Chintakuntlawar AV, Li R, Wei J, Wang L, Liu B, Nowakowski GS, Wang ML, Wang Y. Association of Sex, Age, and Eastern Cooperative Oncology Group Performance Status With Survival Benefit of Cancer Immunotherapy in Randomized Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2012534. [Crossref] [PubMed]


