This article has an erratum available at: http://dx.doi.org/10.21037/qims-2024-02 the article has been update on 2024-06-03 at here.
Whole-body magnetic resonance imaging in two minutes: cross-sectional real-time coverage of multiple volumes
Introduction
Whole-body magnetic resonance imaging (MRI) is an emerging method for screening large portions of the human body. For a recent review of a variety of diagnostic questions see (1). Technical solutions for whole-body scanning either involve multiple conventional (slow) MRI acquisitions or rely on a continuously moving-table approach (2-5). The present proof-of-principle study attempts to speed up pertinent methods by adopting a recent development for rapid coverage of a volume. The method combines cross-sectional real-time MRI with an automatic slice advancement after each frame (6). Whole-body scanning is accomplished by measuring multiple volumes at predefined locations, i.e., fixed table positions, followed by intermediate movements of the patient table. Although the underlying acquisition and reconstruction technique by regularized nonlinear inversion (NLINV) (7) has been shown to be compatible with continuously moving table conditions (8), the proposed method offers several advantageous properties: (I) the data of each cross-sectional image is spatially consistent in slice direction as the patient table remains stationary during data acquisition; (II) each image is largely motion-robust due to acquisition times in the tens of millisecond regime and therefore avoids the need for cardiac gating and allows for free breathing; (III) different body parts may be scanned with optimal sets of receive coils rather than merely the body coil as typically employed for moving table methods; and (IV) each volume acquisition may be freely adjusted with respect to field-of-view, spatiotemporal resolution and contrast. The purpose of this work is to evaluate the technical feasibility of the method and to demonstrate its basic performance in terms of achievable image quality and speed.
Methods
All measurements were performed at 3 T using a conventional MRI system (Magnetom Prisma fit, Siemens Healthineers, Erlangen, Germany). During technical development 8 subjects without known illness were recruited among the students of the local University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and written informed consent was obtained from all subjects prior to MRI. Ethical approval was obtained from the ethics committee of the Goettingen University School of Medicine. Whole-body scanning involved standard vendor receive coils such as a 20-channel head coil for the head and neck region as well as two 18-channel body arrays in combination with suitable elements of a 32-channel spine coil array for thorax and abdomen. Further receive coils are possible when examining even more extended body parts than in this proof-of-concept study.
Whole-body scanning of separate volumes by cross-sectional real-time MRI employs highly undersampled radial fast low angle shot (FLASH) sequences with automatic shifts of the slice position by a fraction of the slice thickness after each frame (6). Joint reconstructions of each image and its set of coil sensitivity maps are performed by solving a nonlinear inverse problem with regularization to the image and coil sensitivity maps of a preceding and partially overlapping frame. The numerical NLINV problem is solved using a Gauss-Newton method with 7 iterative steps (7). Whole-body coverage of multiple slightly overlapping volumes at predefined locations, i.e., at fixed table positions, uses automatic intermediate movements of the patient table in between MRI of stationary volumes. Here, for demonstration purposes, the combination of three such volumes with 20 mm overlap sections allows for scanning a 100 cm volume that covers the body from head to thigh. For experimental details see Table 1. Noteworthy, for future diagnostic applications all parameters are freely selectable. This particularly holds true for the number and size of volumes as well as for the individual field-of-views, resolutions and contrasts. On the other hand, identical parameters for all volumes may support the reformatting of image datasets during a post-processing step.
Table 1
| Study volume | Head | Thorax | Abdomen |
|---|---|---|---|
| Contrast | T2/T1 | T1 | T1 (+ FatSata) |
| Field-of-view, mm2 | 224×224 | 384×384 | 384×384 |
| Image matrix, pixel2 | 320×320 | 320×320 | 320×320 |
| Resolution, mm2 | 0.7×0.7 | 1.2×1.2 | 1.2×1.2 |
| Volume size, mm | 240 | 400 | 400 |
| Frames per volume | 533 | 400 | 400 |
| Slice thickness, mm | 3 | 4 | 4 |
| Slice shift, mm | 0.45 (15%) | 1.00 (25%) | 1.00 (25%) |
| Echo time, ms | 2.39 | 1.66 | 1.66 |
| Repetition time, ms | 4.77 | 2.50 | 2.50 |
| Bandwidth, Hz pixel-1 | 980 | 1,560 | 1,560 |
| Flip angle, degree | 50 | 20 | 20 |
| Spokes per frame | 17 | 21 | 25 |
| Undersampling factorb | 30 | 24 | 20 |
| Time per frame, ms | 81.09 | 52.50 | 62.50 (+13.39a) |
| Time per volume, s | 43.22 | 21.00 | 25.00 (+5.36a) |
a, timings in brackets refer to applications with interleaved fat saturation (FatSat); b, acceleration factors in comparison to fully sampled radial acquisitions. MRI, magnetic resonance imaging.
Numerical reconstructions of the serial multi-coil data are initialized with reference data from a few leading acquisitions with the body coil before switching to the desired set of local coils. These recordings are part of a brief pre-scan period included to ensure steady-state conditions. This strategy avoids problems due to the possible occurrence of phase singularities in multi-coil datasets that in magnitude images lead to “black holes” (9). Moreover, to mitigate any unnecessary computational burden, reconstructions take advantage of a coil compression technique. It relies on a principal component analysis (PCA) that reduces the number of physical coil elements to 8 virtual channels. Unlike static imaging of a fixed slice, a dynamic implementation updates the PCA on a frame-by-frame basis to address spatial variations of coil sensitivity profiles while sweeping the imaging slice through space sensitized by different physical coil elements. To ensure temporal continuity of successive PCA results each new Eigenvector is analyzed and inverted (multiplication by −1) if deemed necessary by comparison to its predecessor. This strategy allows for spatially smooth PCA changes that reflect the corresponding nature of coil sensitivity profiles within a large volume. Finally, images are subject to a modified nonlocal means filter retaining image sharpness (10).
Online NLINV reconstruction is achieved with use of a bypass computer (Sysgen, Bremen, Germany) equipped with 4 graphical processing units (NVIDIA RTX A5000, Santa Clara, CA, USA) and connected to the MRI scanner by a 1 GBit Ethernet link. The procedure is “invisible” to the operator as all sequences are realized in close similarity to vendor protocols. Using homebuild modules the standard reconstruction pipeline (Siemens Healthineers, Erlangen, Germany) sends k-space data to the bypass computer for NLINV reconstruction and receives the resulting image matrices. These images are displayed as regular Digital Imaging and Communications in Medicine (DICOM) images with negligible latency on the scanner and stored in the usual databank1.
Real-time acquisition times for high-resolution images of less than 100 ms are based on radially encoded gradient-echo sequences with a high degree of undersampling (see Table 1 for current factors). Spin-density and T1 contrasts are obtained by spoiled FLASH sequences with randomized RF phases (11), while T2-type contrast or more precisely T2/T1 contrast results from refocused FLASH sequence versions that generate an SSFP free induction decay (FID) signal by rewinding the two radial encoding gradients (12). This ensures a constant gradient moment (i.e., constant phase) for each repetition interval and recovers transverse coherences without problems due to so-called banding artifacts known for fully balanced SSFP sequences with complete rephasing of all three orthogonal gradients (i.e., zero phase). The acquisition parameters used here for preliminary in vivo applications are summarized in Table 1.
For acquisitions with T2-type contrast, it is important to establish proper SSFP conditions. In other words, each spin needs to experience a sufficiently large number of radiofrequency excitations. For example, for motion-robust T2/T1-weighted scans with only a low number of radial spokes (i.e., excitations) per frame, this may be achieved by using a small slice shift to maximize spatial overlap of successive cross-sections (e.g., 15% of the slice thickness). In contrast, the slice shift for applications with T1 (or spin-density) contrast may be larger (e.g., 25% of the slice thickness) and even reach the full slice thickness (i.e., 100%). Accordingly, the measuring times per volume realized here are only 20 to 30 s for a T1-weighted 400 mm body volume, while T2/T1-weighted scans of the head may take up to 50 s for a 240 mm volume. Moreover, supported by very short gradient echo times and the use of refocused FLASH instead of fully balanced SSFP versions, all measurements except those with fat suppression rely on the magnet’s basal homogeneity. Thus no examination time is spent for unnecessary shimming procedures. Finally, neither preparing localizer scans nor any manual planning is required in view of a predefined set of fixed table locations for successively measuring multiple volumes. And because of the short acquisition times for individual frames cardiac or respiratory gating is not necessary.
Since the individual volumes of the proposed whole-body scan represent independent acquisitions, all imaging parameters may be freely set as needed. For example, while head studies often require T2-type (here T2/T1) contrast and high spatial resolution, heart and lung examinations employ T1 contrast at sufficiently high temporal resolution. T1-weighted abdominal scans may add fat suppression (13,14) interleaved with each image acquisition, while angiographic applications may benefit from spatial presaturation (15). On the other hand, if post-processing should involve reformatting of the serial image data from multiple volumes, then all acquisition parameters may be chosen similar to support this desire. It should also be noted that in order to avoid peripheral nerve stimulation and improve patient compliance in future clinical trials, all sequence implementations deliberately use a conservative gradient mode with reduced maximum slew rates (normal mode).
Results
Figure 1 depicts selected transverse images of a T2/T1-weighted brain scan at 0.7 mm in-plane resolution and 3 mm slice thickness, for other parameters see Table 1. The entire sequence of serial images is recorded in cranial direction starting in the neck region. It is obtained in 43 s and shown in Video 1 running at the true acquisition and reconstruction speed (i.e., about 12 fps). Brain studies benefit from both relatively short measuring times to cope with involuntary subject movements during cross-sectional scanning and from T2/T1 contrast to provide optimal sensitivity for pathologies as demonstrated in recent pediatric neuroimaging applications (16).
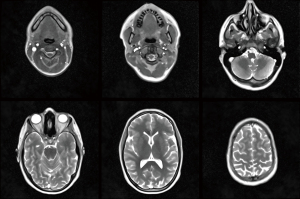
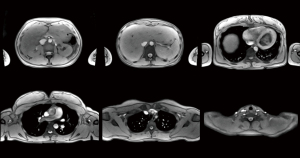
Figure 2 and Video 2 (at 19 fps) show T1-weighted images of the thorax and upper abdomen (for details see Table 1). In order to properly deal with cardiac and respiratory movements within individual cross-sectional images of the heart and lung, emphasis is placed on a sufficiently short measuring time of about 50 ms. This is best accomplished with T1 contrast and spoiled FLASH sequences, whereas refocused FLASH versions suffer from phase discontinuities in the presence of rapidly moving spins (e.g., in the blood pool). Figure 3 and Video 3 (at 13 fps) present T1-weighted images of the abdomen and pelvis with interleaved fat suppression (see Table 1). Such scans are less prone to rapid body movements and therefore allow for slightly longer acquisition times that improve SNR and include extra time for a chemical shift selective (CHESS) fat suppression module (13,14) as a frequent diagnostic option.
Taken together, the example three-volume scanning protocol covering a 100 cm body volume with 20 mm overlap sections and more than 1,300 images leads to a total scan time of less than two minutes including table movements. In case that vendor-controlled procedures for coil adjustments and shimming for fat suppression are required, these may add up to one minute extra time. It should also be noted that the resulting image series of each volume may be used for orthogonal reconstructions. Although at anisotropic resolution and possibly affected by inter-image displacements of organs scanned at different phases of the breathing cycle, such complementary views may turn out insightful in a future diagnostic scenario.
Discussion
This work is a proof-of-principle study of an automatic technique for rapid anatomic whole-body MRI. Typically, total examination times (excluding adjustment procedures) are about two minutes when connecting three volumetric scans in transverse orientation that together cover a 100 cm body volume, for example from head to thigh. Of course, the number and size of volumes per scan is a freely selectable parameter depending on the actual application. Even without cardiac synchronization and during free breathing, the images reveal intra-image motion robustness and provide adequate spatial resolution and tissue contrasts. In other words, the achieved image quality in various body regions promises to be of diagnostic quality. Potential clinical applications are expected to follow the range of diagnostic questions that rely on T1- or T2-weighted whole-body screening for anatomic abnormalities (e.g., cancer) or body composition and may be extended towards peripheral angiography w/wo contrast agent (e.g., vascular disease) (1). A special emphasis may be on applications in pediatric radiology where movements are of particular concern when seeking MRI scanning without sedation or anesthesia (17). However, it is too premature to detail or even prescribe the future clinical usage of the proposed scanning method in this brief technical report. On the other hand, in view of the increasing importance of artificial intelligence in MRI or diagnostic imaging in general, respective applications are expected to play a successful role in the analysis and evaluation of huge image datasets such as generated by the rapid whole-body MRI method presented here.
This study is not without limitations. A major obstacle is the fact that the technique is not yet generally available2. Moreover, this preliminary work only involves healthy volunteers and does not address any diagnostic challenge. Further, the method only offers spin-density, T1 and T2-type contrasts and particularly lacks diffusion contrast which is of high relevance for oncologic questions (18). Moreover, because T2-type contrast is achieved via SSFP sequences, thoracic applications may require fully balanced SSFP versions to minimize the phase effects for rapidly moving spins in T2/T1-weighted refocused FLASH images—though at the risk of banding artifacts. Similarly, abdominal SSFP scans preclude an easy combination with interleaved fat suppression modules as these disturb the steady state of transverse coherences. Finally, three-dimensional reformatting may be compromised by inter-image spatial displacements due to heartbeat and respiration.
Conclusions
This study demonstrates the feasibility and basic performance of a novel method for rapid whole-body MRI with T1 and T2-type contrast. To further evaluate its practical usefulness and diagnostic potential, clinical trials are warranted.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-923/coif). DV and JF report that they are co-inventors of a patent describing the MRI method (Rapid Volume Coverage 2020) used in this study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the ethics committee of the Goettingen University School of Medicine and written informed consent was obtained from all subjects prior to MRI.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
1, for availability of the real-time MRI method, please contact: Jens Frahm, Max Planck Institute for Multidisciplinary Sciences, 37070 Göttingen, Germany. Email: jfrahm@mpinat.mpg.de.
2, For availability contact: Jens Frahm. Max Planck Institute for Multidisciplinary Sciences, 37070 Göttingen, Germany. Email: jfrahm@mpinat.mpg.de.
References
- Summers P, Saia G, Colombo A, Pricolo P, Zugni F, Alessi S, Marvaso G, Jereczek-Fossa BA, Bellomi M, Petralia G. Whole-body magnetic resonance imaging: technique, guidelines and key applications. Ecancermedicalscience 2021;15:1164. [Crossref] [PubMed]
- Hu HH, Madhuranthakam AJ, Kruger DG, Glockner JF, Riederer SJ. Continuously moving table MRI with SENSE: application in peripheral contrast enhanced MR angiography. Magn Reson Med 2005;54:1025-31. [Crossref] [PubMed]
- Fautz HP, Kannengiesser SA. Sliding multislice (SMS): a new technique for minimum FOV usage in axial continuously moving-table acquisitions. Magn Reson Med 2006;55:363-70. [Crossref] [PubMed]
- Boernert P, Aldefeld B. Principles of whole-body continuously-moving-table MRI. J Magn Reson Imaging 2008;28:1-12. [Crossref] [PubMed]
- Sengupta S, Smith DS, Welch EB. Continuously moving table MRI with golden angle radial sampling. Magn Reson Med 2015;74:1690-7. [Crossref] [PubMed]
- Voit D, Kalentev O, van Zalk M, Joseph AA, Frahm J. Rapid and motion-robust volume coverage using cross-sectional real-time MRI. Magn Reson Med 2020;83:1652-8. [Crossref] [PubMed]
- Uecker M, Zhang S, Voit D, Karaus A, Merboldt KD, Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed 2010;23:986-94. [Crossref] [PubMed]
- Zenge MO, Uecker M, Mattauch G, Frahm J. Continuous table movement MRI in a single breath-hold: Highly undersampled radial acquisitions with nonlinear iterative reconstruction and joint coil estimation. Proc Intl Soc Mag Reson Med 2012;20:14.
- Voit D, Kalentev O, Frahm J. Body coil reference for inverse reconstructions of multi-coil data—the case for real-time MRI. Quant Imaging Med Surg 2019;9:1815-9. [Crossref] [PubMed]
- Klosowski J, Frahm J. Image denoising for real-time MRI. Magn Reson Med 2017;77:1340-52. [Crossref] [PubMed]
- Roeloffs V, Voit D, Frahm J. Spoiling without additional gradients: Radial FLASH MRI with randomized radiofrequency phases. Magn Reson Med 2016;75:2094-9. [Crossref] [PubMed]
- Frahm J, Hänicke W. Rapid scan techniques. In: Stark DD, Bradley WG, editors. Magnetic Resonance Imaging. 3rd edition. St. Louis: Mosby; 1999:87-124.
- Haase A, Frahm J, Hänicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol 1985;30:341-4. [Crossref] [PubMed]
- Frahm J, Haase A, Hänicke W, Matthaei D, Bomsdorf H, Helzel T. Chemical shift selective MR imaging using a whole-body magnet. Radiology 1985;156:441-4. [Crossref] [PubMed]
- Frahm J, Merboldt KD, Hänicke W, Haase A. Flow suppression in rapid FLASH NMR images. Magn Reson Med 1987;4:372-7. [Crossref] [PubMed]
- Gräfe D, Roth C, Weisser M, Krause M, Frahm J, Voit D, Hirsch FW. Outpacing movement - ultrafast volume coverage in neuropediatric magnetic resonance imaging. Pediatr Radiol 2020;50:1751-6. [Crossref] [PubMed]
- Hirsch FW, Frahm J, Sorge I, Roth C, Voit D, Gräfe D. Real-time magnetic resonance imaging in pediatric radiology - new approach to movement and moving children. Pediatr Radiol 2021;51:840-6. [Crossref] [PubMed]
- Morone M, Bali MA, Tunariu N, Messiou C, Blackledge M, Grazioli L, Koh DM. Whole-Body MRI: Current Applications in Oncology. AJR Am J Roentgenol 2017;209:W336-49. [Crossref] [PubMed]