
Atypical meningiomas with multiple extracranial metastases: a case description
Introduction
Meningiomas are mostly benign tumors originating from the arachnoid cap cells, with an annual incidence of 5/100,000, which gradually increases with age. It is the most common benign brain tumor of the central nervous system in adults (1). Meningiomas are more common in women, with a male-to-female ratio of approximately 2–3:1. Meningiomas can present with a wide range of clinical symptoms. Although most meningiomas are not malignant, they can cause clinical symptoms when they grow large and compress important areas of the brain or spinal cord. The 2016 World Health Organization (WHO) classification of tumors of the central nervous system classifies meningiomas into 3 types: benign (WHO class I), atypical (WHO class II), and anaplastic/malignant (WHO class III). The revisions of the WHO classification in 2007 produced a marked increase in atypical meningiomas (AMs) from the previously reported 5% (2-4). AMs have biological characteristics that fall between benign and malignant and are highly heterogeneous and invasive. Multiple extra-cranial metastases of AMs are rare, occurring in about 0.1% of cases. Most reported cases of metastasis have been found in the lungs, liver, or spine (5). Only a few similar cases have been documented, involving multiple distant metastases of intracranial meningiomas with extensive pleural metastatic features (6-8). However, it is important to note that these meningiomas are mostly mesenchymal in nature and often accompanied by intracranial recurrences. In this study, we present a rare case of AM with multiple metastases to the thorax, abdomen, and bone, while noting the absence of intracranial recurrence. Additionally, we provide a comprehensive review and discussion of the histopathologic features and mechanisms of metastatic meningiomas. The main objective of this study was to assist clinicians in minimizing misdiagnosis, improving diagnostic clarity, and offering valuable insights for the postoperative management and treatment of AM patients.
Case presentation
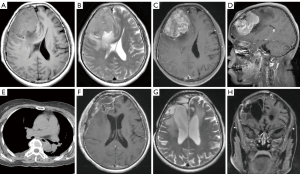
A 64-year-old female patient presented to the Neurosurgery Department of The Second Affiliated Hospital of Nanchang University in September 2018 due to a 3-month history of headache. Brain magnetic resonance imaging (MRI) suggested a possible hemangiopericytoma (Figure 1A-1D). Chest computed tomography (CT) showed no obvious abnormalities (Figure 1E). With the assistance of neuro-navigation, the doctor opened the skull through the right midline frontotemporal flap and saw that the tumor was encroaching on the skull and eroding the capitellar tendon, with poorly defined tumor margins and extremely rich blood supply, and removed it completely after freeing the tumor margins. Postoperative follow-up head MRI showed complete resection of the right frontal lesion (Figure 1F). After discharge, the patient underwent brain MRI scans every 6 months during follow-up visits, and no signs of tumor recurrence were detected (Figure 1G,1H). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
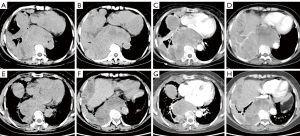
In October 2021, the patient was admitted to the hospital due to persistent chest pain. Enhanced CT of the chest revealed the presence of multiple masses of varying sizes adjacent to the spine and pleura, extending into the abdominal cavity. The enhancement was significantly uneven, and the border between the liver parenchyma and right kidney was blurred (Figure 2A-2D).
Some abnormalities in the combination of lung cancer markers (Table 1) and no abnormalities were found in other electrolytes and routine blood tests. Whole-body bone scan revealed an abnormally active metabolism in the left posterior rib of the 7th and the right anterior ribs of the 7th and 8th vertebrae. The patient’s physical status (PS) score was 1 point, and the numerical rating scale (NRS) was 1 point.
Table 1
| Indicator | Test result | Reference value |
|---|---|---|
| CEA (ng/mL) | 1.23 | 0–5.09 |
| CA-199 (U/mL) | 13.04 | 0–37 |
| SCCA (ng/mL) | 3.24↑ | <2.5 |
| NSE (ng/mL) | 52.13↑ | <10 |
| Cytokeratin 19 fragment (ng/mL) | 1.40 | <3.3 |
↑ indicates that the corresponding indicator is higher than the normal range. CEA, carcinoembryonic antigen; CA-199, carbohydrate antigen-199; SCCA, squamous cell carcinoma antigen; NSE, neuron-specific enolase.
Immunization combined with targeted therapy was used for treatment, with camrelizumab for injection 200 mg day 1, oral administration of anlotinib hydrochloride 10 mg days 1–14, supplemented by symptomatic treatment such as antiemetic and stomach protection. After 6 cycles of treatment, the patient’s symptoms improved and imaging showed a reduction in lesions compared to before surgery, indicating that the treatment was effective (Figure 2E-2H).
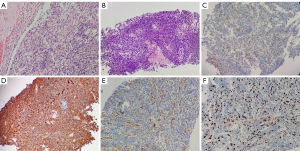
Pathological features: pathological findings of patients after the operation suggested an AM (WHO grade II) (Figure 3A). Puncture of the pleural mass, hematoxylin and eosin (HE)-stained tumor cells were spindle-shaped or oval, arranged in a swirl shape, infiltrative growth, and extensive necrosis (Figure 3B). Immunohistochemical results: the patient’s epithelial membrane antigen (EMA) (Figure 3C), vimentin (Figure 3D), and cluster of differentiation 34 (CD34) (Figure 3E) were all positive, and the Ki-67 proliferation index was high (hot spot >20%) (Figure 3F). Combined with the patient’s right frontal AM medical history, the final pathological diagnosis was metastatic meningioma after biopsy and immuno-omics examination of the pleural lesion.
Discussion
AM is an aggressive form of meningioma, with a recurrence rate of 30–60% at 5 years after surgery, which poses a central and important issue in the management of AM patients (9-12). The pathogenesis of distant metastases from meningiomas is unknown and may be related to the following causes: (I) meningioma tumor cells located in the venous sinus shed into the cortical vein or invade the sagittal sinus, and metastasize to the extracranial through venous blood; (II) surgical resection increases the risk of tumor cell dissemination and implantation along the cerebrospinal fluid pathway; (III) Intraoperative salvage autotransfusion; (IV) metastases involving the spine and kidneys may be related to the metastasis of the paravertebral venous plexus; (V) extracranial metastasis by invading the lymphatic system around the cranial nerve (13-17).
In general, maximal safe resection is the recommended first-line treatment for all meningiomas (18,19). In order to prevent recurrence, Simpson I–II grade resection is often used clinically to completely remove the tumor within a safe range. The patient underwent Simpson I grade resection and postoperative adjuvant radiotherapy, but still had distant metastasis 3 years later. In most cases, patients with AMs should receive adjuvant radiotherapy after surgery; however, some studies have shown that there is no significant difference in progression-free survival whether or not radiotherapy is given after surgery (8,20). It has also been suggested that histopathological criteria could be reconsidered according to risk stratification for stronger prognostic value (i.e., 2 out of 3: absence of psammoma bodies, presence of necrosis, and/or ≥4 mitoses per 10 high-power fields) (21).
In recent years, more research has been conducted on the pathogenesis, development, and treatment of meningioma using molecular techniques, and monosomy 22 and NF2 inactivating mutations are genetic alterations that have been identified (22). Ongoing studies are focused on molecular characterization of gene mutations, such as SMO, TERT, TRAF7, and DNA methylation profiles. Sahm et al. conducted a genome-wide methylation pattern analysis and discovered that different methylation types were significantly correlated with tumor growth and recurrence patterns. They proposed a new classification system for meningiomas based on methylation category (23); however, the sample size of the study was too small and may be heterogeneous. Unfortunately, next-generation DNA/RNA sequencing and methylome analysis have not yet been performed in our patient; we believe that genetic testing can help to determine adjuvant treatment options for patients with meningioma metastases, and we will continue to follow up on the results of the patient’s testing in the future.
The report indicates that metastases exhibited anaplastic characteristics, including cellular pleomorphism, nuclear atypia, and a high Ki-67 index. Immunohistochemistry and molecular pathology methods have been effectively utilized in the grading and typing of meningiomas. The markers EMA and vimentin are preferred auxiliary diagnostic markers for meningioma, specifically for identifying epithelial and mesenchymal tissue. These markers are used to differentiate between different types of meningiomas and aid in diagnosis (24). An immunohistochemical marker alone may not accurately predict prognosis and needs to be used in conjunction with an indicator of cell proliferative activity, and Ki-67 is strongly correlated with the degree of malignancy, metastasis, or prognosis of meningiomas. Barrett reported that AMs with high expression of Ki-67 and mitotically active mitosis were prone to recurrence after surgery (25). The patient in this case report had a pleural metastasis with a Ki-67 hotspot of >20%, indicating that the tumor cells were highly malignant and proliferated rapidly.
In summary, our findings emphasize the importance of careful re-evaluation of patients with a history of intracranial meningeal tumors, especially patients with WHO grade II–III meningiomas, for early detection and treatment of any distant metastases, even if such metastases are rare, with the aim of maximizing the long-term prognosis of the patient. Recommend regular brain MRI to exclude in situ or borderline recurrence, as well as whole-body imaging to identify metastases, and genetic testing is also necessary to determine subsequent treatment options. In addition, the extracranial metastases in this patient were significantly reduced/shrunk with immunotherapy combined with targeted therapy, and future clinical trials, prospective studies, and further molecular studies are needed to reliably determine whether this treatment regimen is an effective treatment for extracranial metastases of meningiomas.
Acknowledgments
The authors thank the numerous individuals who participated in this study, the editors, and the anonymous reviewers for their insightful suggestions on this work.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-565/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother 2018;18:241-9. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, Vecht C. Meningioma. Crit Rev Oncol Hematol 2008;67:153-71. [Crossref] [PubMed]
- Wang KD, Su YB, Zhang Y. Recurrent intracranial meningioma with multiple pulmonary metastases: A case report. Oncol Lett 2015;10:2765-8. [Crossref] [PubMed]
- Honda Y, Shirayama R, Morita H, Kusuhara K. Pulmonary and pleural metastasis of intracranial anaplastic meningioma in a 3-year-old boy: A case report. Mol Clin Oncol 2017;7:633-6. [Crossref] [PubMed]
- Kaminski JM, Movsas B, King E, Yang C, Kronz JD, Alli PM, Williams J, Brem H. Metastatic meningioma to the lung with multiple pleural metastases. Am J Clin Oncol 2001;24:579-82. [Crossref] [PubMed]
- Keric N, Kalasauskas D, Freyschlag CF, Gempt J, Misch M, Poplawski A, Lange N, Ayyad A, Thomé C, Vajkoczy P, Meyer B, Ringel F. Impact of postoperative radiotherapy on recurrence of primary intracranial atypical meningiomas. J Neurooncol 2020;146:347-55. [Crossref] [PubMed]
- Pisćević I, Villa A, Milićević M, Ilić R, Nikitović M, Cavallo LM, Grujičić D. The Influence of Adjuvant Radiotherapy in Atypical and Anaplastic Meningiomas: A Series of 88 Patients in a Single Institution. World Neurosurg 2015;83:987-95. [Crossref] [PubMed]
- Nowak A, Dziedzic T, Krych P, Czernicki T, Kunert P, Marchel A. Benign versus atypical meningiomas: risk factors predicting recurrence. Neurol Neurochir Pol 2015;49:1-10. [Crossref] [PubMed]
- Cao X, Hao S, Wu Z, Wang L, Jia G, Zhang L, Zhang J. Treatment Response and Prognosis After Recurrence of Atypical Meningiomas. World Neurosurg 2015;84:1014-9. [Crossref] [PubMed]
- Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M, Weber DC, Baumert BG, Canyilmaz E, Yalman D, Szutowicz E, Tzuk-Shina T, Mirimanoff RORare Cancer Network. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys 2008;71:1388-93. [Crossref] [PubMed]
- Akimura T, Orita T, Hayashida O, Nishizaki T, Fudaba H. Malignant meningioma metastasizing through the cerebrospinal pathway. Acta Neurol Scand 1992;85:368-71. [Crossref] [PubMed]
- Magu SK, Gupta MK, Behl A. Malignant Meningioma - Clinicopathological Study of an Unusual Presentation of a Rare Malignancy. Med J Armed Forces India 2003;59:166-8. [Crossref] [PubMed]
- Nabeya Y, Okazaki Y, Watanabe Y, Tohnosu N, Yamazaki M, Matsuda M, Iizuka H, Akutsu N, Kono T, Sato H, Kubosawa H. Metastatic malignant meningioma of the liver with hypoglycemia: report of a case. Surg Today 1998;28:953-8. [Crossref] [PubMed]
- He N, Zhong L, Lei K. Multiple Pulmonary and Pleural Metastases in Recurrent Intracranial Meningioma with Genetic Changes: Case Report and Review of the Literature. World Neurosurg 2020;136:337-40. [Crossref] [PubMed]
- Surov A, Gottschling S, Bolz J, Kornhuber M, Alfieri A, Holzhausen HJ, Abbas J, Kösling S. Distant metastases in meningioma: an underestimated problem. J Neurooncol 2013;112:323-7. [Crossref] [PubMed]
- Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, Barani IJ, James CD, Parsa AT. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol 2014;16:628-36. [Crossref] [PubMed]
- Sun SQ, Hawasli AH, Huang J, Chicoine MR, Kim AH. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus 2015;38:E3. [Crossref] [PubMed]
- Kessler RA, Garzon-Muvdi T, Yang W, Weingart J, Olivi A, Huang J, Brem H, Lim M. Metastatic Atypical and Anaplastic Meningioma: A Case Series and Review of the Literature. World Neurosurg 2017;101:47-56. [Crossref] [PubMed]
- Backer-Grøndahl T, Moen BH, Arnli MB, Torseth K, Torp SH. Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol 2014;7:7206-19. [PubMed]
- Yuzawa S, Nishihara H, Tanaka S. Genetic landscape of meningioma. Brain Tumor Pathol 2016;33:237-47. [Crossref] [PubMed]
- Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 2017;18:682-94. [Crossref] [PubMed]
- Abdelzaher E, Abdallah DM. Expression of mesothelioma-related markers in meningiomas: an immunohistochemical study. Biomed Res Int 2014;2014:968794. [Crossref] [PubMed]
- Barrett OC, Hackney JR, McDonald AM, Willey CD, Bredel M, Fiveash JB. Pathologic Predictors of Local Recurrence in Atypical Meningiomas Following Gross Total Resection. Int J Radiat Oncol Biol Phys 2019;103:453-9. [Crossref] [PubMed]


