
Alteration of intracerebral metabolites and subjective sleepiness by acute caffeine administration in adults
Introduction
Several disease processes are associated with abnormal metabolism with additional drug effects on the relevant brain metabolites. Magnetic resonance spectroscopy (MRS) has been available to provide robust, noninvasive, and highly sensitive measurement of brain metabolites since the late 1980s (1). However, the sensitivity of MRS in detecting fluctuations in brain metabolites needs to be better defined. Caffeine is the most widely consumed psychoactive substance in the world to mitigate sleepiness and enhance performance. It is an active ingredient in coffee, tea, and soft drinks as well as products containing cocoa or chocolate, various medications, and dietary supplements (2). A wide variety of health benefits have been attributed to caffeine, including a reduced incidence of chronic and degenerative diseases such as cancer, cardiovascular disorders, diabetes, and Parkinson’s disease (3).
Non-invasive techniques, such as MRS, provide powerful tools for examining the effects of psychoactive substances on brain metabolism in vivo in real time and elucidating their mechanisms of therapeutic action. For example, the J-difference edited MRS method, Mescher-Garwood point resolved spectroscopy (MEGA-PRESS), is widely used to detect γ-aminobutyric acid (GABA), which is a major inhibitory neurotransmitter in the human central nervous system (CNS). Various biological activities of GABA, including anti-hypertensive, anti-diabetic, anti-carcinogenic, antioxidant, anti-inflammatory, anti-microbial, and anti-allergic properties, have been documented (4).
However, since GABA is only present in the cerebral cortex in millimolar levels (1–2 mmol/L), the duplicability and repeatability of MEGA-PRESS at 3 Tesla require further investigation. Coefficients of variation between and within subjects were found to be 10%, suggesting that GABA estimates have good short- and long-term stability and reliability under normal conditions (5). Nonetheless, whether acute caffeine consumption affects brain metabolite levels measured by MRS remains unclear. People with different coffee consumption habit have various brain functional connectivity at rest (6). Astrid Nehlig summarized the benefits and risks of caffeine intake on CNS, which include increased alertness and concentration and prevention of Alzheimer’s and Parkinson’s disease, but it induces anxiety and sleep disturbances, and so on (7). Therefore, it is worthwhile investigating whether acute caffeine consumption would impact the metabolites of brain, and whether the results would differ according to coffee consumption habits.
Although a number of factors have been determined to be the potential source of the additional differences in brain metabolites, few studies have investigated the acute beverage intake such as coffee on MRS metabolite estimates in different brain regions. Moreover, it currently remains unclear whether the habit of coffee consumption affects MRS estimates of brain metabolites. Caffeine is an antagonist of A1 and A2A adenosine receptors. Thus, brain volume changes may be caused by the antagonist binding of caffeine to adenosine receptors (8). Additionally, caffeine may decrease amyloid accumulation in the brain by blocking adenosine receptors to reduce production of white matter hyperintensities (9).
A study that included 398,646 participants from the UK Biobank has provided evidence that high coffee consumption is associated with smaller total brain volumes and increased odds of dementia (10). Another large prospective study reported a J-shaped association between separate coffee consumption and the risk of all-cause mortality. Drinking one cup of coffee per day seems to be linked with the lower risk of morality (11). Paiva et al. reported that conventional caffeine consumption can increase the signal-to-noise ratio in the process of information encoding, which increases the salience of information processing during learning in neural circuits (12). Acute caffeine intake can delay sleep initiation and reduce sleep intensity, however, the daily intake of caffeine every morning and afternoon will not seriously damage the night sleep structure, nor will it damage the subjective sleep quality of the healthy sleepers who often consume caffeine (13). In contrast, chronic caffeine intake induces changes in adenosine levels and/or adenosine receptors, which are related to development of tolerance for physiological and subjective measures such as blood pressure, heart rate, and alertness (14). In late-preterm infants, it appears that caffeine does not act as a CNS stimulant or adversely affect sleep. Moreover, preterm and term infants metabolize caffeine differently from older children and adults (15).
In the present study, the spectroscopic volumes of the thalamus (TH) and posterior cingulate cortex (PCC) were evaluated because these are key cerebral areas for caffeine activity. Moreover, basal ganglia (i.e., putamen, TH, and insula) presents increased activity after caffeine ingestion. The blood-oxygenation-level-dependent activation changes in the basal ganglia after caffeine consumption are additional evidence that it induces high-level cognitive control (16). Particularly, TH has the highest density of A1 receptors in the brain (17), with caffeine’s ability to induce cognitive performance and greater alertness possibly related to its function as an adenosine A1 receptor antagonist (17,18). Alternatively, the PCC may contain nodes of the default mode network, which has been postulated to be related to consciousness and self-awareness and may be involved in the effect of caffeine on vigilance, while the posterior regions of the default mode network may have elevated neural activity due to caffeine use (19,20).
To our knowledge, few studies have monitored alterations in intracerebral metabolite levels in adults during caffeine administration (5). The objective of this study was two-fold: to assess changes in brain metabolites after caffeine consumption, and to identify differences in metabolite levels attributable to caffeine consumption habits. Additionally, we aimed to explore the relationship between changes in metabolite levels and subjective sleepiness. We present this article in accordance with the TREND reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-635/rc).
Methods
Participants
Participants were recruited through self-selection by advertisements on the Xiamen University and Jimei University’s social media between April 2022 and October 2022. GPower was used to perform calculations on sample size and statistical power. The minimal significance (α), statistical power (1−β), and effect size f were set at 0.05, 0.80, and 0.25 respectively. Eighty-two percent power can be achieved when sample size is 36. Forty-five healthy adult participants aged between 19 and 50 years of age (21 men, n=45; mean age: 25.6±8.76 years) were included in the study. The recruitment of participants is shown in Figure 1. The exclusion criteria were the presence of neurological, endocrine, psychiatric disorders or diabetes, habitual consumption of mind-altering substances, woman’s menstrual period, and individuals who were unable to undergo magnetic resonance imaging (MRI) (due to metal implants, pacemaker, neurostimulator, body piercings, or claustrophobia). The participants were instructed to abstain from consuming beverages or substances containing caffeine [including coffee, soft drinks, tea, functional beverages, cocoa, chocolate and medications such as antipain formulations and dietary supplements (7)] for at least 24 h prior to the metabolite and sleepiness assessments. Written informed consent was obtained from each participant before the beginning of the examinations, and the study was approved by the Ethics Department of the Second Affiliated Hospital of Xiamen Medical College and Ethics Committee of Medical College of Xiamen University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
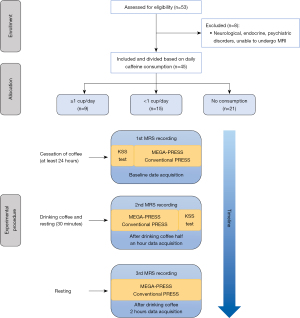
The basic information questionnaire inquired on the daily volume of coffee consumption. If the participants consumed coffee three or more times per week, they were defined as “caffeinated drinks drinkers” (18). The participants were divided into three experimental groups based on their caffeine consumption habits as follows (19): participants who consumed a minimum of one coffee per day (≥1 cup/day) (six men, n=9; mean age: 39.3±10.5 years), participants who consumed less than one cup of coffee per day (<1 cup/day) (six men, n=15; mean age: 20.0±1.2 years), and participants with no regular caffeine consumption habit, considered as “non-coffee drinkers” (NCD) (nine men, n=21; mean age: 23.7±4.1 years). Consumption of coffee and other caffeinated products was confirmed through a structured interview.
MRI and MRS protocols
All MRI and MRS examinations were performed with one clinical 3.0-T MRI scanner (Discovery MR750w, GE Healthcare, Milwaukee, WI, United States) with a 24-channel head coil. MRI examination was performed at baseline and 30 and 120 min after the participants consumed coffee. Specifically, 30 min was as a time interval chosen based on previous neuroimaging investigations of caffeine effects on blood flow and brain activity (5,16,20). Tmax of caffeine ranged from 77 to 115 min (21). Therefore, in order to balance the scan and rest time, we chose to operate the third scan at 120 min. We considered any MRI scan that took place within the half-life of caffeine to be an “acute intake”. The Karolinska Sleepiness Scale (KSS) assessment was performed at the time of the baseline MRS recording and 90 min after coffee consumption. Figure 1 illustrates a diagram of the study protocol. Prior to spectroscopy, we acquired axial T2-weighted fast spin echo (FSE) images [repetition time (TR) =3,500 ms, echo time (TE) =100 ms, number of excitations (NEX) =2, field of view (FOV) =240 mm, slice thickness =5 mm, acquisition time =2 min 6 s], coronal T2 FLAIR images (TR =9,000 ms, TE =145 ms, NEX =1, FOV =240 mm, slice thickness =5 mm, acquisition time =2 min 52 s), and sagittal T2 FLAIR images (TR =9,000 ms, TE =145 ms, NEX =1, FOV =240 mm, slice thickness =5 mm, acquisition time =1 min 57 s). High-resolution MRI was performed using a three-dimensional (3D) BRAVO sequence (BRAin VOlume) (TR =7.0 ms, TE =2.4 ms, NEX =1, FOV =240 mm, flip angle =12°, slice thickness =1 mm).
Placement of MRS volumes was based on anatomical location. The first spectroscopic volume (TH, “TH voxel”) was placed on the right side of the third ventricle (Figure 2A). The second spectroscopic volume (PCC, “PCC voxel”) was placed in the central parietal lobe (Figure 2B). As much cortex as possible was included within each voxel and unwanted lipid contamination from the skull was avoided in all cases. Routine MRS was obtained in the transverse plane using a volume pre-selected PRESS hybrid sequence as follows: directions of the voxel of interest (VOI) = anterior-posterior (AP) × left-right (LR) × foot-head (FH) =20 mm × 20 mm × 20 mm (8 mL), TR =3,000 ms, TE =35 ms; 128 averages; acquisition time =7 min 36 s (Figure 2C). We collected two spectra from the TH and PCC voxels over 14 min. The scan parameters for MEGA-PRESS were: VOI = AP × LR × FH =20 mm × 30 mm × 30 mm (18 mL); TR =1,800 ms, TE =68 ms; spectral width =2,000 Hz; 2,048 data points; 160 averages; acquisition time =10 min 19 s (Figure 2D). Two spectra were collected over a 20-min period. The editing frequency was 1.7 ppm for “On” (editing frequency, −356 Hz) and 7.7 ppm for “Off” (editing frequency, 356 Hz) spectra; the editing pulse shape was set to 12. Due to the frequency of 1.7 ppm, macromolecular resonance was partially affected by “On” spectra, resulting in a notable contribution of macromolecules (MM) signal to the 3-ppm peak. Thus, all GABA estimates were referred as “GABA + MM” (GABA+) in this manuscript (5). The shimming quality was set to achieve a full width at half maximum (FWHM) linewidth of 14 Hz of the water peak.

KSS and caffeine intake
Subjective sleepiness was measured before and 90 min after the consumption of caffeine using the KSS, which assessed participants’ momentary state of alertness/sleepiness on a scale of 1–9 (“extremely alert” to “extremely sleepy”) (22).
KSS has previously been used to demonstrate sensitivity to caffeine effects (23). Commercial coffee (Starbucks cold brewed coffee) was used as the caffeine-containing drink; one cup of cold brewed coffee contained 200 mg of caffeine.
Participants were required to drink the coffee within 5 minutes.
Post-processing and image analysis
Spectra were coiled with weighting factors derived from the first point of the non-water-suppressed induction decay signal. Quantitation of metabolites in k-space was performed in the frequency domain. Fourier-transformed (processed) free induction decay (FID) consists of signals in absorption mode, which are defined by their specific resonance frequency, line shape, line width, and phase (24). FID can be modeled as a sum of sinusoids decaying exponentially. There are three model functions for FID in frequency domain: Lorentzian [exp (−αt)], Gaussian [exp (−βt2)] and Voigt [exp (−αt −βt2)] line shapes (25). Fourier transformation is a mathematical method for decomposing signals into their constituent frequencies. The pre-processed MRS data can be analyzed as a linear combination (LC) of “model” in vitro spectra from individual metabolite solution. A “best fit” is produced by the linear combination of the model (LCModel, Stephen Provencher Inc., Oakville, ON, Canada) when overlapping peaks are analyzed (26). Based on the edited spectra, we calculated the water-scaled concentrations of metabolites using the LCModel and the simulated basis set for GABA+, glutamate + glutamine (Glx), glutamate (Glu), glycerylphosphocholine and phosphocholine (GPC + PCH), myo-inositol (Ins), and creatine and phosphocreatine (Cr + PCr).
LCModel analysis (Version 6.3-1L) was conducted using the control parameter sptype = “MEGA-PRESS-2” for GABA+ quantification. Due to factors affecting signal intensity in MEGA-PRESS, such as J-coupling effects and T2 losses, absolute values should be viewed as institutional units rather than absolute values because they may have an additional (unknown) scaling factor.
For arbitrating the spectra of absolute metabolite concentrations, the lower bounds of Cramer-Rao were used as the primary guideline. Our study included only metabolite spectra with LCModel uncertainty estimates of 15% standard deviation (SD) or greater, and with a signal-to-noise ratio >3. Our method of estimating line shape and baseline accounted for residual water signals using a constrained regularization method. The study excluded spectra with a FWHM of >15 Hz.
Statistical analyses
The normal distribution of metabolite levels was tested with a one-sample Kolmogorov-Smirnov test within each spectroscopic volume (TH and PCC) and each condition (pre-coffee, 30 min post-coffee, and 120 min post-coffee). Two-way repeated-measures analysis of variance (ANOVA) was performed to assess the effects of the within-subject factor of time (pre-coffee vs. 30 min post-coffee vs. 120 min post-coffee), and the between-subject factor of groups (1 cup/day vs. 1 cup/day vs. NCD). Age was used as a covariate to correct the results. In ANOVA, two-way interactions (time × group) were of interest. In the absence of any interaction effects, the main effects for times and groups were analyzed; otherwise, simple effects were analyzed. Bonferroni’s multiple comparison test was used for the subsequent pairwise comparisons. Bivariate correlation analysis was used to determine the correlation between brain metabolite levels and subjective sleepiness. Pearson’s correlation coefficient was typically used for jointly normally distributed data. For non-normally distributed data, a Spearman’s Rank Order Correlation was used as a measure of a monotonic association. All statistical analyses were performed using SPSS Statistics Package (version 25.0; IBM Corporation, New York, NY, United States). All tests were two sided and P<0.05 was considered statistically significant. All the pictures were drawn using GraphPad Prism 8 (GraphPad Software, San Diego, USA).
Results
Group and time comparison analysis
Baseline information of the participants are shown in Table S1. Two-way repeated-measures ANOVA was used to examine whether metabolites in the brain changed over the course of the study for ≥1 cup/day, <1 cup/day, and NCD groups. The results are presented in detail in Table S2 and Table S3.
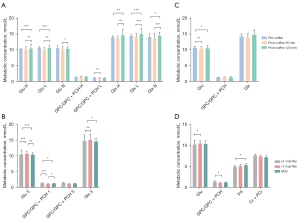
There was a significant time × group interaction effect among the metabolic concentrations in the TH voxel in all three periods: Glu (F=3.818; P=0.007), GPC/GPC + PCH (F=5.000; P=0.001), Ins (F=4.950; P=0.001), and Cr + PCr (F=5.375; P=0.001), Glx (F=5.222; P=0.001) (Table S2). Further examination of simple effects revealed time-significant effects across the ≥1 cup/day, <1 cup/day, and NCD groups’ metabolic concentrations in the TH voxel (Figure 3A). Glu decreased in participants in the NCD group 30 min after coffee consumption, and significantly increased to baseline [F=4.821; P=0.013; 95% confidence interval (CI): −1.223 to −0.140]. GPC/GPC + PCH significantly increased in participants in the <1 cup/day (F=16.787; P<0.0001; 95% CI: −0.212 to −0.071) and NCD groups (F=7.983; P=0.001; 95% CI: −0.127to −0.026) 30 min after coffee consumption, and subsequently decreased to baseline. Ins significantly increased in participants of the ≥1 cup/day (F=3.343; P=0.045; 95% CI: 0.023 to 1.043), <1 cup/day (F=20.709; P<0.001; 95% CI: −0.771 to −0.238) and NCD groups (F=9.019; P=0.001; 95% CI: −0.52 to −0.136) 30 min after coffee consumption, but decreased after 120 min. Cr + PCr levels significantly increased in the <1 cup/day group (F=5.238; P=0.01; 95% CI: −0.477 to −0.063) 30 min after coffee consumption, but significantly decreased in the NCD group (F=4.866; P=0.013; 95% CI: 0.026 to 0.231). Glx levels significantly decreased in the ≥1 cup/day group 120 min after coffee consumption (F=5.269; P=0.009; 95% CI: 0.466 to 4.742), and significantly increased in the <1 cup/day group 30 min after coffee consumption (F=3.463; P=0.041; 95% CI: −2.701 to −0.057). Significant simple effects on metabolic concentrations in the TH voxel by group across the pre-coffee, post-coffee 30 min, and post-coffee 120 min conditions are presented in Figure 3B. Glu was significantly lower in the ≥1 cup/day group than in the <1 cup/day and NCD groups at the pre-coffee (F=3.795; P=0.031; 95% CI: −2.898 to 0.345), and 120 min post-coffee (F=18.197; P<0.0001; 95% CI: −3.774 to −0.820) points. GPC/GPC + PCH was significantly higher in the ≥1 cup/day group than in the <1 cup/day and NCD groups at the pre-coffee (F=13.156; P<0.001; 95% CI: 0.265 to 0.782), 30 min post-coffee (F=4.216; P=0.022; 95% CI: 0.026 to 0.604), and 120 min post-coffee (F=7.071; P=0.002; 95% CI: −0.038 to 0.504) points. Cr + PCr was significantly higher in the <1 cup/day group than in the ≥1 cup/day and NCD groups at the 30 min post-coffee (F=6.199; P=0.004; 95% CI: −0.501 to −0.055), and 120 min post-coffee (F=31.418; P<0.001; 95% CI: 0.271 to 0.845) points. Ins was significantly higher in the <1 cup/day group than in the NCD group at the 120 min post-coffee (F=5.551; P=0.007; 95% CI: 0.133 to 0.929) point. Glx was significantly higher in the ≥1 cup/day group than in the <1 cup/day and NCD group at the 120 min post-coffee (F=15.661; P<0.001; 95% CI: −5.113 to −1.401) point. Additionally, a significant time effect was observed in the TH voxel for GABA+ (F=6.573; P=0.011), GPC/GPC + PCH (F=4.959; P=0.012), Ins (F=5.272; P=0.009), and Glx (F=7.296; P=0.002) independent of groups (Figure 3C). Similarly, a significant time-independent group effect was seen in the TH voxel for Glu (F=10.123; P<0.0001), GPC/GPC + PCH (F=8.781; P=0.001), Cr + PCr (F=6.867; P=0.003), and Glx (F=4.760; P=0.014) (Figure 3D).

There was a significant time × group interaction effect among the concentrations of Glu (F=8.671; P<0.0001), GPC/GPC + PCH (F=3.240; P=0.016) and Glx (F=7.299; P<0.0001) in the PCC voxel at all three time points (Table S3). Further examination of simple effects revealed time-significant effects across the ≥1 cup/day, <1 cup/day, and NCD groups’ metabolite concentrations in the PCC voxel (Figure 4A). Glu decreased significantly in participants in the ≥1 cup/day group (F=20.678; P<0.0001; 95% CI: 1.012 to 2.573) 120 min after coffee consumption and increased significantly in participants in the <1 cup/day (F=25.359; P<0.0001; 95% CI: −1.135 to −0.245) and NCD (F=4.699; P=0.015) groups 120 min after coffee consumption. Moreover, GPC increased significantly in participants in the <1 cup/day group (F=9.093; P=0.001; 95% CI: −0.729 to −0.079) 30 min after coffee consumption, then decreased slightly. Glx significantly decreased in participants in the ≥1 cup/day group 120 min after coffee consumption (F=9.006; P=0.001; 95% CI: 0.482 to 4.209), and significantly increased in participants in the <1 cup/day (F=21.973; P<0.0001; 95% CI: −2.887 to −0.763) and NCD (F=14.657; P<0.0001; 95% CI: −1.613 to −0.083) group 120 min after coffee consumption. Significant simple effects of the group on metabolic concentration in the PCC voxel across the three time points are shown in Figure 4B. Glu was significantly lower in the ≥1 cup/day group than in the <1 cup/day and NCD groups at the 120 min post-coffee (F=17.921; P<0.0001; 95% CI: −3.84 to −1.576). GPC/GPC + PCH was significantly higher in the ≥1 cup/day group than in the <1 cup/day and NCD groups at the pre-coffee (F=6.390; P=0.004; 95% CI: 0.084 to 0.516), and 120 min post-coffee (F=3.393; P=0.43; 95% CI: −0.011 to −0.456) points. Glx was significantly lower in the ≥1 cup/day group than in the <1 cup/day and NCD groups at the 120 min post-coffee (F=7.206; P=0.002; 95% CI: −6.092 to −1.229) points. A significant time effect in the PCC voxel for Glu (F=40.748; P<0.0001), GPC/GPC + PCH (F=22.808; P<0.0001), and Glx (F=16.539; P<0.0001) was found to be group-independent (Figure 4C). Similarly, a significant group effect in the PCC voxel for Glu (F=3.920; P=0.028), GPC/GPC + PCH (F=4.002; P=0.026), Ins (F=5.263; P=0.009), and Cr + PCr (F=3.637; P=0.035) was observed, and this was independent of time (Figure 4D).
Bivariate correlation analysis between the metabolites in the brain and subjective sleepiness
To assess potential associations between metabolite levels and subjective sleepiness after coffee consumption, Pearson’s or Spearman’s Rank Order Correlation analysis was conducted for the TH and PCC voxels separately. In the TH voxel, the GABA+ concentration difference (post-coffee 30 min value minus pre-coffee value) was negatively correlated with the KSS difference (post-coffee value minus pre-coffee value) (r=−0.7676; P<0.0001; 95% CI: −0.866 to −0.612) (Figure 5). For the remaining metabolites, and all the metabolites in the PCC voxel, there were no significant correlations between metabolite differences and KSS differences (Table 1).

Table 1
| Metabolites | 30 min post-coffee | 120 min post-coffee | |||
|---|---|---|---|---|---|
| r values | P values | r values | P values | ||
| TH | |||||
| GABA+ | −0.7676* | <0.0001* | 0.054 | 0.881 | |
| Glu | 0.296 | 0.351 | 0.070 | 0.828 | |
| GPC/GPC + PCH | 0.018 | 0.955 | −0.015 | 0.964 | |
| Ins | −0.259 | 0.417 | −0.022 | 0.945 | |
| Cr + PCr | −0.067 | 0.837 | −0.159 | 0.622 | |
| Glx | 0.180 | 0.577 | −0.007 | 0.982 | |
| PCC | |||||
| GABA+ | −0.322 | 0.334 | −0.584 | 0.059 | |
| Glu | 0.192 | 0.550 | 0.207 | 0.519 | |
| GPC/GPC + PCH | 0.551 | 0.064 | 0.495 | 0.102 | |
| Ins | −0.351 | 0.263 | −0.351 | 0.263 | |
| Cr + PCr | 0.055 | 0.864 | 0.148 | 0.647 | |
| Glx | −0.018 | 0.955 | −0.107 | 0.740 | |
Bivariate correlation analysis. The table shows that the changes of the above six metabolites in thalamus and posterior cingulate cortex at different time points correlate with the values of the KSS difference. *, statistically significant results (P<0.05). Two-tailed P values are depicted. KSS, Karolinska Sleepiness Scale; TH, thalamus; GABA+, γ-aminobutyric acid-positive macromolecule; Glu, glutamate; GPC, glycerylphosphocholine; PCH, phosphocholine; Ins, myo-inositol; Cr, creatine; PCr, phosphocreatine; Glx, glutamate and glutamine; PCC, posterior cingulate cortex.
Discussion
In this study, the cerebral GABA+ levels of 45 participants were assessed in vivo using MEGA-PRESS before and after oral administration of a 200 mg dose of caffeine. In addition, routine brain MRS levels were analyzed for other major contributors, such as Glu, GPC/GPC + PCH, Ins, Cr + PCr, and Glx. We divided the participants into three groups according to their coffee consumption habits and evaluated the effects of habitual coffee consumption on brain metabolites.
This study found that metabolite levels significantly changed 30 min after ingestion of caffeine, where GABA+ decreased, GPC/GPC + PCH increased, Glx increased, and Ins increased in the TH voxel; Glu decreased, GPC/GPC + PCH increased, and Glx decreased in the PCC voxel; while a significant increase in Glx was seen in the PCC voxel after 120 min. However, these changes were inconsistent with the findings from Oeltzschner et al.’s study (5), which found that GABA+, Glu, and Glx did not show significant differences before and after caffeine intake, while Ins was slightly lower after caffeine intake in the anterior cingulate and occipital areas. This indicates that metabolite concentrations in different brain regions vary significantly before and after caffeine consumption. Caffeine modulates many neurotransmitter systems, including acetylcholine, glutamine, and GABA, with the overall effect of reducing inhibition and increasing activity (27). Caffeine alters the release of GABA, acetylcholine, and Glu via the blockade of A2A receptors; inhibits Glu release for neuroprotection. Remarkably, caffeine may also play a role in the recovery of cognitive function in patients with traumatic brain injury with glutamatergic neuron damage (28). Caffeine has been shown to inhibit GABA release by activating cyclic nucleotide-gated Ca2+ permeable channels (29) and depress GABA-A receptor activity in ganglion cells of the turtle retina (30). GPC enables cells to osmotically adapt and protect cellular MM against denaturing under extracellular hypertonic stress. Caffeine increases cellular GPC content in cultured Madin-Darby Canine Kidney (MDCK) cells and MDCK epithelial cells (31). Cerebral Ins concentration is a marker of glial cell activation and proliferation (32), and astrocytes release Ins to compensate for increased cellular osmolarity. Caffeine may influence cerebral osmoregulation, a mechanism with pivotal involvement in Ins metabolism. Additionally, Cr and caffeine are among the most widely available and used compounds by competitive and recreational athletes (33), for being able to improve strength and sprint performance; however, caffeine ingestion may blunt the ergogenic effect of Cr (34). In a previous study, caffeine was neuroprotective in an animal model of Parkinson’s disease. In contrast, higher caffeine intake was associated with more rapid clinical progression of Parkinson’s disease in participants taking Cr (35).
Regarding caffeine consumption habits, we found the ≥1 cup/day group to be significantly different from the <1 cup/day and NCD groups in the levels of Ins in the PCC voxel and Glu, GPC and Cr + PCr in both voxels. Participants in ≥1 cup/day had a lower concentration of Ins (in the PCC voxel), Glu, Glx, and Cr + PCr (in the TH voxel), and a higher concentration of Cr + PCr and GPC (in both voxels) than those in <1 cup/day and NCD groups. In vivo, the most important physiological function of GPC is to cross the blood-brain barrier and provide the choline necessary for acetylcholine and phospholipid synthesis. Acetylcholine is an important neurotransmitter in the CNS; which helps the brain perform learning, memory, and cognitive activities, and control light sleep and motor activity (36). Previous study has demonstrated the beneficial effects of caffeine on cognitive function, as functional connectivity is reorganized toward more efficient network properties after coffee consumption, highlighting that caffeine may enhance cognition through GPC (37). Cr is an important neuroprotective agent that increases the survival rate of nerve cells during an external attack. Moreover, energy metabolism and reactive oxides are thought to combat the symptoms of neurodegenerative diseases and creatine improves brain function (38). Coffee and its components have several neuroprotective properties that reduce the risk of cognitive decline and other neurodegenerative diseases (39).
Finally, GABA+ levels in the TH voxel were found to be negatively correlated with subjective sleepiness. This correlates with caffeine’s known ability to reduce sleepiness, prolong sleep latency, and enhance the wake period after sleep onset (13). Moreover, GABA is a major inhibitory neurotransmitter in the CNS and GABAergic neurons are a model used to elucidate the mechanisms of rapid eye movement (REM) sleep. REM-on neurons excite GABAergic interneurons that send inhibitory projections to REM-off neurons, which contributes to the generation of REM sleep (40). Previous study has confirmed the relationship between GABA and sleepiness, and the GABA-A receptor antagonist flumazenil normalizes vigilance metrics in patients with hypersomnia associated with abnormal potentiation of GABA receptors (41).
Nonetheless, there are some limitations with this study. Future study designs could feature control groups with a placebo instead of caffeine. In addition, other scales can be applied to measure subjective sleepiness and alterations.
Conclusions
The present study revealed that GABA+ levels in TH voxels significantly correlate with subjective sleepiness. GABA+, GPC/GPC + PCH, Ins, Glu, and Glx levels were significantly altered after caffeine consumption. The levels of Glu, GPC, Cr + PCr, Glx, and Ins were significantly influenced by caffeine consumption habits. These findings illustrate the sensitivity of MRS to fluctuations in brain metabolites and suggest that higher consumption of coffee and caffeinated products have an impact on brain metabolites.
Acknowledgments
The authors thank the volunteers who participated in this study.
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-635/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-635/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Department of the Second Affiliated Hospital of Xiamen Medical College and Ethics Committee of Medical College of Xiamen University. Written informed consent was obtained from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hyder F, Rothman DL. Advances in Imaging Brain Metabolism. Annu Rev Biomed Eng 2017;19:485-515. [Crossref] [PubMed]
- Korekar G, Kumar A, Ugale C. Occurrence, fate, persistence and remediation of caffeine: a review. Environ Sci Pollut Res Int 2020;27:34715-33. [Crossref] [PubMed]
- Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A. Coffee: biochemistry and potential impact on health. Food Funct 2014;5:1695-717. [Crossref] [PubMed]
- Ngo DH, Vo TS. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019;24:2678. [Crossref] [PubMed]
- Oeltzschner G, Zöllner HJ, Jonuscheit M, Lanzman RS, Schnitzler A, Wittsack HJ. J-difference-edited MRS measures of γ-aminobutyric acid before and after acute caffeine administration. Magn Reson Med 2018;80:2356-65. [Crossref] [PubMed]
- Magalhães R, Picó-Pérez M, Esteves M, Vieira R, Castanho TC, Amorim L, Sousa M, Coelho A, Fernandes HM, Cabral J, Moreira PS, Sousa N. Habitual coffee drinkers display a distinct pattern of brain functional connectivity. Mol Psychiatry 2021;26:6589-98. [Crossref] [PubMed]
- Nehlig A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol Rev 2018;70:384-411. [Crossref] [PubMed]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 2008;57:121-34. [Crossref] [PubMed]
- Ritchie K, Artero S, Portet F, Brickman A, Muraskin J, Beanino E, Ancelin ML, Carrière I. Caffeine, cognitive functioning, and white matter lesions in the elderly: establishing causality from epidemiological evidence. J Alzheimers Dis 2010;20:S161-6. [Crossref] [PubMed]
- Pham K, Mulugeta A, Zhou A, O'Brien JT, Llewellyn DJ, Hyppönen E. High coffee consumption, brain volume and risk of dementia and stroke. Nutr Neurosci 2022;25:2111-22. [Crossref] [PubMed]
- Chen Y, Zhang Y, Zhang M, Yang H, Wang Y. Consumption of coffee and tea with all-cause and cause-specific mortality: a prospective cohort study. BMC Med 2022;20:449. [Crossref] [PubMed]
- Paiva I, Cellai L, Meriaux C, Poncelet L, Nebie O, Saliou JM, et al. Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription. J Clin Invest 2022;132:e149371. [Crossref] [PubMed]
- Weibel J, Lin YS, Landolt HP, Kistler J, Rehm S, Rentsch KM, Slawik H, Borgwardt S, Cajochen C, Reichert CF. The impact of daily caffeine intake on nighttime sleep in young adult men. Sci Rep 2021;11:4668. [Crossref] [PubMed]
- Conlay LA, Conant JA, deBros F, Wurtman R. Caffeine alters plasma adenosine levels. Nature 1997;389:136. [Crossref] [PubMed]
- Seppä-Moilanen M, Andersson S, Kirjavainen T. Caffeine is a respiratory stimulant without effect on sleep in the short-term in late-preterm infants. Pediatr Res 2022;92:776-82. [Crossref] [PubMed]
- Park CA, Kang CK, Son YD, Choi EJ, Kim SH, Oh ST, Kim YB, Park CW, Cho ZH. The effects of caffeine ingestion on cortical areas: functional imaging study. Magn Reson Imaging 2014;32:366-71. [Crossref] [PubMed]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol 2000;362:364-74. [Crossref] [PubMed]
- Nerurkar PV, Gandhi K, Chen JJ. Correlations between Coffee Consumption and Metabolic Phenotypes, Plasma Folate, and Vitamin B12: NHANES 2003 to 2006. Nutrients 2021;13:1348. [Crossref] [PubMed]
- Lu MY, Lai JC, Chen SJ. Influence of Sex Differences on Serum Lipid Profiles among Habitual Coffee Drinkers: Evidence from 23,072 Taiwan Biobank Participants. Nutrients 2023;15:2576. [Crossref] [PubMed]
- Wu WC, Lien SH, Chang JH, Yang SC. Caffeine alters resting-state functional connectivity measured by blood oxygenation level-dependent MRI. NMR Biomed 2014;27:444-52. [Crossref] [PubMed]
- Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol Biochem Behav 1997;58:721-6. [Crossref] [PubMed]
- Laverde-López MC, Escobar-Córdoba F, Eslava-Schmalbach J. Validation of the Colombian version of the Karolinska sleepiness scale. Sleep Sci 2022;15:97-104. [Crossref] [PubMed]
- Mednick SC, Cai DJ, Kanady J, Drummond SP. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav Brain Res 2008;193:79-86. [Crossref] [PubMed]
- in 't Zandt H, van Der Graaf M, Heerschap A. Common processing of in vivo MR spectra. NMR Biomed 2001;14:224-32. [Crossref] [PubMed]
- Mandal PK. In vivo proton magnetic resonance spectroscopic signal processing for the absolute quantitation of brain metabolites. Eur J Radiol 2012;81:e653-64. [Crossref] [PubMed]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672-9. [Crossref] [PubMed]
- Sun H, Gonzalez F, McQuillen PS. Caffeine Restores Background EEG Activity Independent of Infarct Reduction after Neonatal Hypoxic Ischemic Brain Injury. Dev Neurosci 2020;42:72-82. [Crossref] [PubMed]
- Lusardi TA, Lytle NK, Gebril HM, Boison D. Effects of Preinjury and Postinjury Exposure to Caffeine in a Rat Model of Traumatic Brain Injury. J Caffeine Adenosine Res 2020;10:12-24. [Crossref] [PubMed]
- Isokawa M. Caffeine-Induced Suppression of GABAergic Inhibition and Calcium-Independent Metaplasticity. Neural Plast 2016;2016:1239629. [Crossref] [PubMed]
- Yoshimura H. The potential of caffeine for functional modification from cortical synapses to neuron networks in the brain. Curr Neuropharmacol 2005;3:309-16. [Crossref] [PubMed]
- Kim DK, Jung KY. Caffeine causes glycerophosphorylcholine accumulation through ryanodine-inhibitable increase of cellular calcium and activation of phospholipase A2 in cultured MDCK cells. Exp Mol Med 1998;30:151-8. [Crossref] [PubMed]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013;8:576-93. [Crossref] [PubMed]
- Elosegui S, López-Seoane J, Martínez-Ferrán M, Pareja-Galeano H. Interaction Between Caffeine and Creatine When Used as Concurrent Ergogenic Supplements: A Systematic Review. Int J Sport Nutr Exerc Metab 2022;32:285-95. [Crossref] [PubMed]
- Trexler ET, Smith-Ryan AE. Creatine and Caffeine: Considerations for Concurrent Supplementation. Int J Sport Nutr Exerc Metab 2015;25:607-23. [Crossref] [PubMed]
- Simon DK, Wu C, Tilley BC, Lohmann K, Klein C, Payami H, Wills AM, Aminoff MJ, Bainbridge J, Dewey R, Hauser RA, Schaake S, Schneider JS, Sharma S, Singer C, Tanner CM, Truong D, Wei P, Wong PS, Yang T. Caffeine, creatine, GRIN2A and Parkinson's disease progression. J Neurol Sci 2017;375:355-9. [Crossref] [PubMed]
- Levillain O, Schmolke M, Guder WG. Influence of dehydration on glycerophosphorylcholine and choline distribution along the rat nephron. Pflugers Arch 2001;442:218-22. [Crossref] [PubMed]
- Kim H, Kang SH, Kim SH, Kim SH, Hwang J, Kim JG, Han K, Kim JB. Drinking coffee enhances neurocognitive function by reorganizing brain functional connectivity. Sci Rep 2021;11:14381. [Crossref] [PubMed]
- Wyss M, Braissant O, Pischel I, Salomons GS, Schulze A, Stockler S, Wallimann T. Creatine and creatine kinase in health and disease--a bright future ahead? Subcell Biochem 2007;46:309-34. [Crossref] [PubMed]
- Wasim S, Kukkar V, Awad VM, Sakhamuru S, Malik BH. Neuroprotective and Neurodegenerative Aspects of Coffee and Its Active Ingredients in View of Scientific Literature. Cureus 2020;12:e9578. [Crossref] [PubMed]
- Wang YQ, Liu WY, Li L, Qu WM, Huang ZL. Neural circuitry underlying REM sleep: A review of the literature and current concepts. Prog Neurobiol 2021;204:102106. [Crossref] [PubMed]
- Rye DB, Bliwise DL, Parker K, Trotti LM, Saini P, Fairley J, Freeman A, Garcia PS, Owens MJ, Ritchie JC, Jenkins A. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med 2012;4:161ra151. [Crossref] [PubMed]


