
A case description of pulmonary enteric adenocarcinoma with KRAS G12D mutation
Introduction
Pulmonary enteric adenocarcinoma (PEAC) is a rare subtype of adenocarcinoma, representing approximately 0.6% of all primary lung adenocarcinomas (1). To date, about 347 cases have been reported in the literature, mostly as case reports or small case series (2). PEAC refers to the intestinal-type differentiation or intestinal-type morphology of primary lung adenocarcinoma. When this component exceeds 50% of the tumor and adenocarcinoma of digestive tract origin is excluded, it is named PEAC (3). PEAC was first defined by Tsao and Fraser in 1991 (4) and was subsequently proposed by the World Health Organization (WHO) classification in 2015 (5). A large proportion of patients with PEAC are men, with a mean patient age of 60–72 years. The main clinical manifestations were respiratory symptoms such as cough, bloody sputum, chest tightness, and asthma, and gastrointestinal symptoms do not appear throughout the course of the disease. Up to now, only nearly 200 cases have been reported. Due to the lack of large clinical studies, there is no effective standard treatment. The main treatment options are surgery and systematic chemotherapy, with fewer reports having described immune checkpoint inhibitor (ICI) therapy. This paper discusses the clinical diagnosis of a case of PEAC and treatment with immunotherapy and chemotherapy.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The patient was a 63-year-old man who presented to our hospital in June 2021 with “cough and expectoration for 1 week”. He was seen at local hospital before went to our hospital. The patient had no history of smoking. A computed tomography (CT) scan showed soft tissue nodules in the lower lobe of the right lung, about 2.8 cm × 2.1 cm in size, with short burrs surrounding (Figure 1). Technetium-based bone scintigraphy showed radiation concentration in the right 4th anterior rib. Combined with CT, the possibility of bone metastasis was considered. Percutaneous pulmonary puncture biopsy was performed at local hospital, and postoperative pathology revealed lung adenocarcinoma.
According to the patient’s medical history, physical signs, laboratory examination, and puncture pathology, the primary diagnosis was stage IV adenocarcinoma of the right lower lung (rib metastasis). The patient expressed a strong desire to undergo surgery. After comprehensive consideration, the patient underwent thoracoscopic radical resection of right lower lung cancer under general anesthesia in the Department of Cardiothoracic Surgery of our hospital on 17 June 2019. Postoperative pathology showed invasive adenocarcinoma in the right lower lobe of the lung, and the histological grade was medium-low differentiation (Figure 2). Visceral pleural invasion and vascular invasion were positive. No metastasis was found in lymph nodes. A right rib excision specimen was found to be metastatic poorly differentiated carcinoma with the same origin as the lung lesion. Immunohistochemistry (IHC) assays showed that the tumor was positive for CK7, focally positive for CDX2 and villin, and negative for NapsinA, TTF1, and CK20 (Figure 3). Expression of programmed cell death ligand 1 (PD-L1; 22C3) was 10%. Subsequently, the patient’s gastric intestinal endoscope was done and the result was negative. Based on the patient’s medical history, clinical manifestations, pathological morphology, immunophenotype, and gastric intestinal endoscope, the final diagnosis was enteric adenocarcinoma of the right lower lung (pT3N0M1), stage IV. Next-generation sequencing (NGS) found there were no common driver mutations, such as EGFR mutation, ALK, ROS1, BRAF, METex14 skipping, but revealed positivity for a KRAS mutation (p.G12D). However, most KRAS mutations still lack effective target inhibitory drugs.
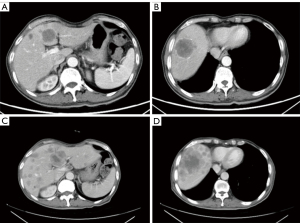
Sintilimab combined with pemetrexed and cisplatin was selected as the first-line regimen, and a total of 3 cycles of chemotherapy were performed from 30 July 2019 to 12 October 2019. CT review was performed on 3 November 2021 for efficacy evaluation. Contrast-enhanced CT revealed multiple newly discovered circular low-enhancement foci in the liver, with a larger diameter of 4.7 cm (Figure 4A,4B). According to Response Evaluation Criteria In Solid Tumors (RECIST) Version 1.1 (RECIST 1.1), the efficacy was evaluated as progressive disease (PD). Given the heterogeneity of the tumor, we suggested that the patient undergo percutaneous liver biopsy to reconfirm driver gene mutation status, but the patient refused. At that time, our cancer center was developing a clinical trial numbered NCT04921358. The patient was successfully enrolled in the clinical study, and randomized into Group B, with docetaxel (75 mg/m2 IV q3w). After 3 cycles of docetaxel treatment, CT was performed on 5 January 2022. Unfortunately, the liver metastases were shown to have enlarged again (Figure 4C,4D). We recommended percutaneous liver biopsy, but the patient refused again. Anlotininb was selected as the subsequent third-line treatment. A total of 3 cycles of targeted therapy were performed from 8 January 2022 to 3 March 2022. As a consequence of the coronavirus disease, the patient did not come to our hospital for treatment. We received the news of the patient’s death on 14 May 2022.
Discussion
PEAC was classified as an independent variant by the International Multi-Scientific Classification of Lung Adenocarcinoma in 2011 (5). PEAC retains the structural features of primary lung adenocarcinoma but has unique IHC characteristics. At least 1 of the gut-derived markers such as CDX2, CK20, MUC2, and villin could be positively expressed. Pulmonary markers CK7, TTF-1, and Napsin A could be partially positive or completely negative. Nottegar et al. (6) analyzed 46 cases of PEAC and showed that the positive rates of CK7 and CDX2 were 100%, whereas the positive rates of TTF1, CK20, and MUC2 were 45.6%, 32.6%, and 32.6%, respectively. Therefore, it was interpreted that CK7 combined with CDX2 is an important marker for PEAC. Cases of PEAC with all negative lung cancer-specific immunophenotypes have also been reported (7). In terms of molecular characteristics, PEAC has a higher probability of KRAS gene mutation, but there have been no common driver genes mutation identified, such as EGFR, ALK, ROS1, and MET. In 46 PEAC cases of the molecular detection, Nottegar et al. (6) found that KRAS 12 codon mutation rate was up to 60.9% (28/46), and EML4-ALK rearrangement rate was up to 13.0% (6/46). EGFR exon 19 deletion mutations was present in only 1 patient. Mutation sites included KRAS G12D, G12V, G12C, and G13D, among others. At present, there is no strong consensus on the IHC characteristics of PEAC, so the diagnosis and differential diagnosis should not be overly reliant on IHC expression. Clinical history, imaging, pathological morphology, and IHC results should be comprehensively assessed. In the present case, IHC assays and molecular characteristics conformed to the diagnosis of PEAC.
At present, there is no standard treatment for PEAC. Traditional non-small cell lung cancer (NSCLC) treatment is recommended instead of colorectal cancer treatment. Due to the special structure of KRAS protein, except for the GTP/GDP binding pocket, the surface is smooth and relatively shallow, so it is difficult to target KRAS therapeutic drugs. Therefore, platinum-based chemotherapy has long been the first choice in the treatment of KRAS-mutated lung cancer, but the efficacy of chemotherapy is not ideal. Sotorasib and MRTX849, the G12C inhibitors of KRAS, have shown promising antitumor activity. Among 124 patients with locally advanced or metastatic NSCLC who had disease progression after receiving ICIs and/or platinum-based chemotherapy, the objective response rate (ORR) of sotorasib was 36%, the disease control rate (DCR) was 81%, and the median response duration was 10 months. The duration of response (DOR) was ≥6 months in 58% of patients. Based on the CodeBreaK100 Clinical Trials (8), the Food and Drug Administration (FDA) announced accelerated approval of sotorasib for patients with locally advanced or metastatic NSCLC with the KRAS G12C mutation who have received at least 1 previous systemic therapy. In the published clinical trial data of the phase II trial KRYSTAL-1 (NCT03785249), adagrasib (9) had an ORR of up to 45%. Nevertheless, it our case, the KRAS mutation site of the patient was G12D, for which there is still no effective target inhibitory drug.
In 2019, Mazieres et al. (10) conducted a retrospective analysis of patients with advanced NSCLC who received ICI monotherapy and had at least 1 oncogenic driver change (8), and found that among 551 patients from 24 centers, the ORR of KRAS mutation patients in immune-related therapy was 26%,which is higher than that of other types of driver gene mutations. Therefore, immunotherapy may be effective against KRAS mutations. This was also confirmed in multiple subsequent clinical studies. In the KEYNOTE-042 clinical trial (11), pembrolizumab monotherapy compared with chemotherapy significantly prolonged progression-free survival (PFS) [hazard ratio (HR) =0.51, 12 vs. 6 months] in patients with KRAS mutations. Patients with KRAS-G12C mutation appeared to benefit more (HR =0.27, 15 vs. 6 months). A meta-analysis (12) examined randomized trial data comparing first- or second-line anti-PD-L1 (IMpower-150, Keynote-189, Keynote-042, Oak, Poplar, and CheckMate-057) with or without chemotherapy versus chemotherapy alone for advanced KRAS-mutant NSCLCs. For KRAS-mutant NSCLCs, anti-PD-L1 with or without chemotherapy was significantly associated [HR (95% confidence interval)] with prolonged OS [0.59 (0.49–0.72); P<0.00001] and PFS [0.58 (0.43–0.78); P=0.0003)] compared to chemotherapy alone. Overall survival (OS) benefited in both first- and second-line trials.
Based on the above research results, immunotherapy combined with platinum-based chemotherapy was used as the first-line treatment. Unfortunately, this patient’s PFS1 was only 3 months. In view of tumor heterogeneity, needle biopsy and NGS gene sequencing of new liver metastases were recommended to search for possible therapeutic targets; however, the patient refused. Thus, we suggested that the patient be enrolled in a “Randomized Phase 3 Study of Tislelizumab in Combination With Sitravatinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer that Progressed on or After Platinum-based Chemotherapy and Anti-PD-L1 Antibody” (SGB-A317-Sitravatinib-301). This is an open clinical study, and the patient was enrolled to the docetaxel alone group. Unfortunately, the patient did not benefit more from this regimen than he did from the first-line regimen. Anlotinib was selected as the subsequent third-line treatment, but the patient did not display a clear benefit.
In summary, this is a therapeutic observation of immunotherapy combined with chemotherapy with PEAC patient harboring a KRAS G12D mutation. Only a few cases have reported the association between immunotherapy and PEAC. The findings observed in this patient may lead to studies on the treatment and prognosis of patients with PEAC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-369/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu W, Chan CM, Gong L, Bui MM, Han G, Letson GD, Yang Y, Niu X. Malignancy in giant cell tumor of bone in the extremities. J Bone Oncol 2021;26:100334. [Crossref] [PubMed]
- Fassi E, Mandruzzato M, Zamparini M, Bianchi S, Petrelli F, Baggi A, Alberti A, Grisanti S, Berruti A. Clinical presentation and outcome of patients with enteric-type adenocarcinoma of the lung: A pooled analysis of published cases. Lung Cancer 2023;179:107176. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G, Garg K, Austin JH, Rusch VW, Hirsch FR, Jett J, Yang PC, Gould MAmerican Thoracic Society. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer 1991;68:1754-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Nottegar A, Tabbò F, Luchini C, Brunelli M, Bria E, Veronese N, Santo A, Cingarlini S, Gilioli E, Ogliosi C, Eccher A, Montagna L, Pedron S, Doglioni C, Cangi MG, Inghirami G, Chilosi M. Pulmonary Adenocarcinoma With Enteric Differentiation: Immunohistochemistry and Molecular Morphology. Appl Immunohistochem Mol Morphol 2018;26:383-7. [Crossref] [PubMed]
- Matsushima J, Yazawa T, Suzuki M, Takahashi Y, Ota S, Nakajima T, Yoshino I, Yokose T, Inoue T, Kawahara K, Nakatani Y. Clinicopathological, immunohistochemical, and mutational analyses of pulmonary enteric adenocarcinoma: usefulness of SATB2 and β-catenin immunostaining for differentiation from metastatic colorectal carcinoma. Hum Pathol 2017;64:179-85. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N Engl J Med 2022;387:120-31. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G. KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Landre T, Justeau G, Assié JB, Chouahnia K, Davoine C, Taleb C, Chouaïd C, Duchemann B. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunother 2022;71:719-26. [Crossref] [PubMed]





