
Endovascular re-canalization for symptomatic non-acute intracranial large artery occlusion: a single-center retrospective study
Introduction
A significant etiology of stroke is large intracranial artery occlusion, which has been linked to a high risk of stroke recurrence and poor outcomes, particularly among the East Asian population (1-3). Multiple large-scale clinical studies have provided evidence of the effectiveness and safety of endovascular treatment (EVT) for acute large vessel occlusive stroke (4-6). As a result, EVT has now become a recognized first-line treatment option for patients with cerebral infarction (7-9). The utilization of EVT in patients with non-acute large arterial occlusion is still a subject of debate and controversy.
Despite aggressive drug treatment, numerous patients with symptomatic non-acute intracranial large artery occlusion (SNA-ILAO) within 48 hours to 6 months still experience recurrent ischemic events (10-12). Chronic inadequate cerebral blood flow can result in cognitive dysfunction (13). However, the optimal treatment approach for SNA-ILAO remains a subject of debate (14). To address this issue, we conducted a retrospective study of 24 patients with SNA-ILAO who underwent endovascular recanalization. Our goal was to evaluate the feasibility, safety, and effectiveness of this treatment approach. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-643/rc).
Methods
Study design and population
SNA-ILAO refers to complete occlude of an intracranial artery caused by atherosclerosis, leading to relative symptoms (10-12). Endovascular therapy is performed at least 48 hours after the patient experiences symptoms. In this study, we conducted a retrospective review of our stroke intervention database to identify consecutive patients who underwent endovascular re-canalization for atherosclerotic SNA-ILAO between 2018 and 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board approved this study. Written informed consent from patients was waived for this retrospective analysis.
The study’s inclusion criteria were based on four factors: (I) confirmation of intracranial large artery total occlusion through digital subtraction angiography (DSA) of the internal carotid artery (ICA), middle cerebral artery (MCA) M1-3 segments, and vertebrobasilar artery; (II) intracranial atherosclerosis as the primary etiology of the occlusion; (III) patients who experienced recurrent transient ischemic attacks (TIAs) or strokes and neurological deterioration, which could be caused by intracranial large artery occlusion despite aggressive medical treatment (AMT); (IV) confirmation of hemodynamic failure and hypo-perfusion in the intracranial large artery territory based on clinical and imaging evidence.
The study’s exclusion criteria were as follows: (I) occlusion caused by conditions other than atherosclerosis, such as arterial dissection or embolic disease; (II) patients with stable clinical symptoms undergoing AMT; (III) individuals with known allergies or contraindications to medication, such as anti-platelet or anesthesia, making surgery unsafe; (IV) patients or their family members explicitly declining endovascular re-canalization.
Clinical and radiological assessment
We evaluated patient demographic information and comorbidities in this study. Experienced neurologists assessed the modified Rankin scale (mRS) scores and the National Institutes of Health Stroke Scale (NIHSS) scores to determine stroke severity. The mRS is a 6-point disability scale with possible scores ranging from 0 to 5 (15). The NIHSS is a standardized scoring tool used by physicians and other healthcare professionals to measure and record the level of impairment caused by a stroke (16). We first assessed the presence of intracranial large artery total occlusion using non-invasive methods. The diagnosis was later confirmed by DSA. Total occlusion was defined as grade 0 antegrade flow distal to the occlusion according to the thrombolysis in cerebral infarction (TICI) grading system observed during DSA.
Intervention procedure
Before the endovascular recanalization procedures, we routinely administered dual antiplatelet treatment consisting of 100 mg aspirin and 75 mg clopidogrel daily for at least 3 days (17). Thromboelastography platelet mapping was performed to ensure optimal modulation of antiplatelet treatment. We conducted comprehensive evaluation of the occlusion course and collateral circulation through DSA. All procedures were performed under general anesthesia.
In order to access the occluded artery, a 0.014 Synchro micro-guidewire was employed with the assistance of an Excelsior SL-10 soft micro-catheter. The micro-guidewire was meticulously navigated through the occluded lesion to the distal segment and then removed. Subsequently, the length of the occlusion and the distal lumen of the lesion were confirmed through micro-catheter angiography. For cases with an excessively tortuous vessel pathway, a distal access catheter was employed. The lesions were initially treated with conventional balloons. The decision to use stenting was at the operator’s discretion. In instances where the residual stenosis exceeded 50% and antegrade perfusion was unstable, or when vessel dissection occurred following balloon angioplasty, remedial stenting implantation was opted. If clear clots were present at or around the occlusion lesions, an intravenous low dose tirofiban injection was administered (18).
The degree of antegrade flow through the previously occluded artery was evaluated using the TICI grading system. Technical success was determined by achieving re-canalization with a TICI grade of ≥2b on post-procedural angiography. Following the procedure, a cerebral computed tomography (CT) scan was performed immediately, and patients were transferred to the intensive care unit where their blood pressure was strictly monitored. The TICI grading system was also utilized to evaluate the antegrade flow after the procedure, and technical success was defined as achieving a TICI grade of ≥2b on post-procedural angiography (11). The occurrence of any complications during the procedure was recorded.
Follow-up outcomes
During discharge, all patients were prescribed dual anti-platelet therapy, which consisted of both aspirin (100 mg) and clopidogrel (75 mg). The duration of treatment varied based on the procedure performed, with angioplasty patients required to continue the regimen for 3 months and stenting patients for 6 months. Following this period, patients were advised to continue with one of the two drugs.
The patients in this study underwent clinical follow-up at one month, and all patients were clinically followed up again at 1 or 2 years. They were scheduled for a DSA procedure between three to six months after initial treatment. A favorable functional outcome was defined as mRS score of 0 to 2 [1]. Restenosis was defined as a diameter of the stenosis that exceeded 50% of the target artery segment (19). Symptomatic re-stenosis was defined as restenosis accompanied by ischemic symptoms within the treated vessel territory (20).
Statistical analysis
Continuous data were presented as either the mean ± standard deviation (SD) or the median with the interquartile range (IQR), while categorical data were presented as numbers and percentages. SPSS version 25.0 for Windows was used for statistical analysis.
Results
This study enrolled a total of 24 patients, with the majority being male (14/24, 58.3%). Table 1 presents the admission details for all patients, and Table 2 outlines the baseline demographics of SNA-ILAO patients. The patients’ mean age was 62.0±9.3 years. Hypertension (16/24, 66.7%), smoking (13/24, 54.2%), coronary artery disease (10/24, 41.7%), diabetes mellitus (7/24, 29.2%), hyperlipidemia (6/24, 25.0%), and atrial fibrillation (3/24, 12.5%) were the most common risk factors. The study found that the pre-treatment median mRS and NIHSS scores were 3 (IQR, 1–4) and 1 (IQR, 1–3), respectively.
Table 1
| Case | Age (years) | Gender | Occlusion location | Symptom onset to treatment (days) | Pre-treatment TICI degree | Pre-treatment NIHSS score | Treatment modalities | Post-treatment TICI degree | Complication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | Female | L-MCA M2 | 3 | 0 | 3 | BD | 3 | – |
| 2 | 59 | Female | L-MCA M1 | 12 | 1 | 2 | BD | 3 | – |
| 3 | 59 | Female | L-MCA M2 | 4 | 1 | 11 | BD | 3 | – |
| 4 | 57 | Male | L-MCA M2 | 5 | 1 | 4 | BD | 3 | – |
| 5 | 61 | Male | R-ICA C3 | 6 | 0 | 1 | BD + stent | 3 | ICH |
| 6 | 74 | Male | L-MCA M1 | 3 | 1 | 5 | BD | 3 | – |
| 7 | 49 | Male | R-MCA M1 | 2 | 1 | 5 | BD + stent | 3 | – |
| 8 | 73 | Male | L-MCA M1 | 7 | 1 | 6 | BD + stent | 3 | – |
| 9 | 44 | Female | L-MCA M1 | 10 | 0 | 3 | BD + stent | 3 | – |
| 10 | 68 | Male | L-MCA M1 | 14 | 0 | 6 | BD | 2b | – |
| 11 | 59 | Male | L-MCA M1 | 3 | 1 | 4 | BD | 3 | – |
| 12 | 56 | Male | L-MCA M1 | 14 | 1 | 4 | BD | 3 | – |
| 13 | 44 | Female | R-MCA M1 | 6 | 1 | 0 | BD | 3 | – |
| 14 | 68 | Female | R-MCA M2 | 10 | 0 | 3 | BD | 3 | ICH |
| 15 | 50 | Male | R-MCA M2 | 5 | 0 | 3 | BD + stent | 3 | – |
| 16 | 68 | Female | R-MCA M1 | 30 | 0 | 0 | BD + stent | 2b | – |
| 17 | 70 | Male | L-MCA M1 | 3 | 1 | 2 | BD + stent | 3 | – |
| 18 | 64 | Male | L-MCA M1 | 90 | 0 | 1 | BD | 3 | – |
| 19 | 66 | Male | L-MCA M1 | 30 | 0 | 1 | BD | 3 | – |
| 20 | 48 | Female | R-MCA M1 | 14 | 0 | 2 | BD + stent | 3 | – |
| 21 | 68 | Male | L-MCA M1 | 7 | 0 | 3 | BD | 3 | – |
| 22 | 69 | Male | L-MCA M4 | 14 | 0 | 0 | BD | 3 | – |
| 23 | 70 | Female | R-MCA M1 | 20 | 0 | 1 | BD + stent | 2b | – |
| 24 | 71 | Female | L-MCA M1 | 6 | 0 | 0 | BD + stent | 3 | – |
TICI, thrombolysis in cerebral infarction; NIHSS, the National Institutes of Health Stroke Scale; L, left; MCA, middle cerebral artery; BD, balloon dilatation; R, right; ICA, internal carotid artery; ICH, intracerebral hemorrhage.
Table 2
| Variables | Value (n=24) |
|---|---|
| Age (years) | 62.0±9.3 |
| Male | 14 (58.3) |
| Pre-treatment NIHSS | 3 [1–4] |
| Pre-treatment mRS score | |
| 0 | 5 (20.8) |
| 1 | 14 (58.3) |
| 2 | 4 (16.7) |
| 3 | 1 (4.2) |
| Comorbidities | 14 (58.3) |
| Hypertension | 16 (66.7) |
| Diabetes mellitus | 7 (29.2) |
| Hyperlipidemia | 6 (25.0) |
| Atrial fibrillation | 3 (12.5) |
| Coronary artery disease | 10 (41.7) |
| Smoking history | 13 (54.2) |
Data are presented as n (%) or mean ± SD [range]. NIHSS, the National Institutes of Health Stroke Scale; mRS, modified Rankin scale.
EVT
Table 3 summarizes the treatment modalities and their details. The median time from symptom onset to EVT was 10 days (IQR, 6–40 days), and the median time from image-documented intracranial large artery total occlusion to EVT was 9 days. The MCA was the most common occlusion location, including M1 (70.8%), M2 (20.8%), and M3 (4.7%). Successful re-canalization was achieved in all 24 patients, with 21 cases (87.5%) achieving TICI 3 re-perfusion and the remaining 3 cases (12.5%) achieving TICI 2b re-perfusion. Stents were used following conventional balloon dilatation to inhibit intimal hyperplasia in 10 cases (41.7%). The residual stenosis after the procedure was 12.5%.
Table 3
| Variables | Value |
|---|---|
| Symptom onset to treatment (days) | 10 [6–40] |
| Image-documented occlusion to treatment (days) | 9 [6–14] |
| Technical success | 24 (100.0) |
| Occlusion location | |
| MCA M1 | 17 (70.8) |
| MCA M2 | 5 (20.8) |
| MCA M4 | 1 (4.7) |
| ICA C3 | 1 (4.7) |
| Pre-procedural perfusion | |
| TICI 1 | 10 (41.7) |
| TICI 0 | 14 (58.3) |
| Modality of re-canalization | |
| BD | 14 (58.3) |
| BD + stent | 10 (41.7) |
| Post-procedural perfusion | |
| TICI 3 | 21 (87.5) |
| TICI 2b | 3 (12.5) |
| Residual stenosis | 3 (12.5) |
| Complication rate | 2 (8.3) |
| Asymptomatic ICH | 2 (8.3) |
| Perforating branch occlusion | 0 |
| Dissection | 0 |
Data are presented as n (%) or median [interquartile range]. MCA, middle cerebral artery; ICA, internal carotid artery; TICI, thrombolysis in cerebral infarction; BD, balloon dilatation; ICH, intracerebral hemorrhage.
Clinical and angiographic follow-up data
Two patients (8.3%) experienced complications related to the procedure, but there were no cases of perforating branch occlusion or symptomatic dissection. However, two patients (8.3%) experienced asymptomatic ICH after the procedure. Hemorrhaging was found in the previous occipital lobe infarction, which could have been caused by hyperperfusion after recanalization. Nevertheless, it is crucial to note that there were no other periprocedural complications in this case series, such as subarachnoid hemorrhage or perforation. Table 4 presents the clinical and angiographic follow-up outcomes of patients who were successfully treated. Patients who underwent successful recanalization experienced a rapid improvement in their lifestyle-limiting symptoms within a few days. The rate of symptom improvement after the procedure was 75.0% (18/24) for these patients. During the first 30-day clinical follow-up, none of these patients experienced any recurrent cerebral ischemic events. Furthermore, a favorable clinical outcome (mRS score ≤2) was achieved by 88.3% (20/24) of the patients, while 87.5% (21/24) of the patients achieved an acceptable clinical outcome (mRS score ≤3). Twenty-three out of 24 patients achieved a favorable clinical outcome with an mRS score of ≤2, which accounted for 95.8% of the total patients. Additionally, all 24 patients achieved an acceptable clinical outcome with an mRS score of ≤3 at the last follow-up, making it a 100% success rate.
Table 4
| Variables | Data |
|---|---|
| Follow-up time (months) | 29.5 [21–30] |
| Symptom improved post-procedure | 18 (75.0) |
| 30-day mRS score <2 | 20 (88.3) |
| 30-day mRS score <3 | 21 (87.5) |
| mRS score at last follow-up <2 | 23 (95.8) |
| mRS score at last follow-up <3 | 24 (100.0) |
| Ischemic event during follow-up | 0 |
| Death | 0 |
| Restenosis on follow-up imaging | 3 (12.5) |
Data are presented as n (%) or median [interquartile range]. mRS, modified Rankin scale.
Over the median 29.5 months (IQR, 12–45 months) follow-up period for vessel imaging, 8 patients (33.3%) underwent DSA, while 16 patients (66.7%) underwent CTA. Angiographic follow-up in these patients indicated ongoing clot dissolution after successful endovascular recanalization. Restenosis was observed in only 12.5% (3/24) of patients who underwent follow-up imaging. During the clinical follow-up period, no cases of recurrent stroke were observed in patients who had successful recanalization.
Illustrative cases
Case 1
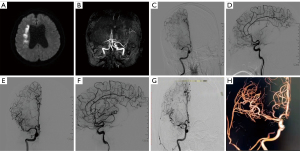
A 57-year-old woman with a medical history of hypertension, diabetes, and hyperlipidemia presented with left-sided weakness and numbness that had persisted for more than 3 months. Magnetic resonance imaging (MRI) revealed an infarct in the right basal ganglia region (Figure 1A). Magnetic resonance angiography (MRA) did not visualize the right MCA (Figure 1B). DSA showed that the distal end of the right MCA was not visualized, and blood flow was slow. Chronic occlusion of the right cerebral subartery was suspected (Figure 1C,1D). After consultation with family members, balloon dilatation was performed. Postoperative DSA showed restoration of blood flow in the distal ICA and MCA, with a normal parent artery (Figure 1E,1F). After 2 years of treatment, DSA and three-dimensional imaging showed a normal MCA and distal blood flow, and no stent stenosis was observed (Figure 1G,1H).
Case 2
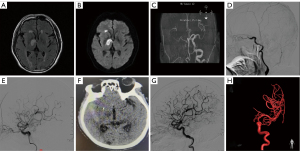
A 61-year-old man with a medical history of hypertension, hyperlipidemia, and a smoking habit of 1 pack per day presented with left hemiplegia that had persisted for more than 1 month. MRI revealed an infarct in the right basal ganglia region (Figure 2A,2B). MRA did not visualize the right ICA (Figure 2C). DSA showed that the distal part of the right ICA was not visualized, and blood flow was slow. Chronic ICA occlusion was suspected (Figure 2D). After family discussion, balloon dilation and stent implantation were performed. Postoperative DSA showed that blood flow in the distal ICA and MCA had been restored, with a normal parent artery (Figure 2E). The patient developed severe headache on the second day after the operation, and CT examination revealed a high-density shadow in the right temporal lobe. Considering postoperative hyperperfusion hemorrhage, the patient was treated conservatively (Figure 2F). One year after treatment, DSA and three-dimensional imaging showed a normal ICA and distal blood flow, with no stent stenosis observed (Figure 2G,2H).
Discussion
The present study provides evidence that endovascular recanalization is a viable option for treating SNA-ILAO, with acceptable perioperative procedure-related morbidity and satisfactory level of safety. Our findings suggest that successful endovascular recanalization can effectively improve disability in patients with SNA-ILAO.
Previous research indicates that intracranial arterial occlusion is responsible for a substantial proportion of stroke cases and TIA cases in China, resulting in high recurrence rates and mortality (9,21). Despite intensive drug treatment, many patients with SNA-ILAO fail to effectively improve their symptoms of recurrent ischemic stroke. Moreover, the effectiveness of extracranial-intracranial artery bypass grafting in patients with SNA-ILAO and hemodynamic impairment is still unclear, as there is a lack of large-scale clinical studies demonstrating its efficacy. Notably, the incidence of recurrent stroke in refractory patients may be higher than previously reported in randomized trials, suggesting that endovascular recanalization remains a viable option (10,22). Our study demonstrates that endovascular recanalization is a viable, successful, and safe method for treating SNA-ILAO, with the potential to alleviate patient symptoms and reduce the short-term recurrence rate of TIA or stroke.
The success and complication rates of EVT for SNA-ILAO are influenced by various factors, including lesion location, vascular diameter, occlusion length, angle, and surgeon experience (9). Currently, it is generally accepted that endovascular recanalization is appropriate for M1-2 segments of the MCA and 6–7 segments of the intracranial ICA in cases of intracranial large artery occlusion (23). Compared to endovascular recanalization of distal MCA occlusion, proximal MCA occlusion and distal ICA occlusion have a better clinical prognosis. According to expert consensus, endovascular recanalization is suitable for vascular diameters greater than 2 mm and occlusion lengths less than 15 mm (16,24). Imaging examinations can determine the location, diameter, occlusion length, and angle of the occluded vessel. In this study, we successfully recanalized 24 patients with varying degrees of TICI grade, including 3 cases of grade 2b and 21 cases of grade 3. We observed asymptomatic ICH in 2 cases (8.3%) as perioperative complications, but no reperfusion syndrome occurred after the operation. Furthermore, there were no perioperative deaths. The technical success rate of this study is consistent with numerous reports on endovascular recanalization of intracranial large artery occlusion (25,26).
The endovascular recanalization of SNA-ILAO has been associated with serious clinical hazards, including bleeding after recanalization, hyperperfusion syndrome, arterial dissection, and distal vascular embolization (27). These complications are frequently encountered during the procedure and can pose significant health risks. Vascular perforation and reperfusion injury caused by guide wires are the major causes of bleeding following vascular recanalization, with an incidence rate of 0.3–5%. Experienced surgeons can mitigate the occurrence of complications such as perforation (28,29). However, in our study, we reported two cases of postoperative bleeding, which is slightly higher than the incidence rate reported in previous studies. This could be attributed to poor control of postoperative blood pressure. It has been demonstrated that the duration of treatment significantly affects the reduction of perioperative complications associated with intracranial artery stenosis and occlusion (30,31). Moreover, studies have revealed that stent placement within 14 days of an ischemic stroke caused by intracranial atherosclerotic artery occlusion may elevate the risk of stroke recurrence and restenosis (9,22,32). Furthermore, patients with ischemic stroke who underwent extracranial-intracranial bypass surgery within 7 days had a higher risk of perioperative stroke than those who underwent surgery after 7 days (33).
The present study suggests that ICA is a more suitable candidate for balloon angioplasty as compared to MCA due to its simpler vascular structure. The complex vascular structure of MCA, such as severe distortions at the end of ICA and proximal occlusion, can pose technical challenges for stent placement and increase the risk of stent thrombosis and restenosis. This notion is supported by previous research (34,35). While stent placement in M1 segment has been associated with a higher risk of stroke, balloon angioplasty alone can be a feasible alternative that reduces the risk of bleeding complications and the need for prolonged use of dual antiplatelet drugs. In our study, balloon dilatation alone demonstrated comparable results in terms of residual stenosis and ischemic events during follow-up, indicating its viability as a standalone treatment option.
Our investigation has shown that endovascular recanalization is a feasible, secure, and efficient therapeutic choice for non-acute intracranial large artery occlusion with symptoms. This intervention has the potential to relieve symptoms in patients and decrease the incidence of TIA or stroke in the short-term.
Our study has certain limitations. Firstly, it was a retrospective study and did not include a comparison between this cohort and SNA-ILAO patients without acute atherosclerosis who did not undergo re-canalization. Therefore, it is necessary to conduct large randomized controlled trials to verify the noninvasive nature of re-canalization. Second, the limited sample size of follow-up imaging data in patients may restrict the assessment of the overall re-stenosis and re-occlusion rate. Thus, high-level clinical evidence in support of using endovascular re-canalization to treat symptomatic non-acute intracranial large artery occlusion is presently insufficient. Additional prospective and multi-center studies are required to validate the safety, efficacy, and long-term benefits of this therapy.
Conclusions
Recanalization has shown promising results as a safe and effective procedure for patients with symptomatic non-acute intracranial large artery occlusion. However, it is important to note that the procedure carries a non-negligible risk of complications. Therefore, it is essential to exercise caution and implement rigorous controls when administering the procedure to patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-643/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-643/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, Gonzalez G, Schaefer PW, Dillon WP, Koroshetz WJ, Furie KL. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009;40:3834-40. [Crossref] [PubMed]
- Yamauchi H, Higashi T, Kagawa S, Kishibe Y, Takahashi M. Chronic hemodynamic compromise and cerebral ischemic events in asymptomatic or remote symptomatic large-artery intracranial occlusive disease. AJNR Am J Neuroradiol 2013;34:1704-10. [Crossref] [PubMed]
- Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002;33:1857-62.
- Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N Engl J Med 2023;388:1272-83. [Crossref] [PubMed]
- Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, et al. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. N Engl J Med 2022;387:1361-72. [Crossref] [PubMed]
- Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N Engl J Med 2020;382:1981-93. [Crossref] [PubMed]
- Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, et al. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. N Engl J Med 2022;387:1373-84. [Crossref] [PubMed]
- Alemseged F, Nguyen TN, Coutts SB, Cordonnier C, Schonewille WJ, Campbell BCV. Endovascular thrombectomy for basilar artery occlusion: translating research findings into clinical practice. Lancet Neurol 2023;22:330-7. [Crossref] [PubMed]
- Rodrigues FB, Neves JB, Caldeira D, Ferro JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ 2016;353:i1754. [Crossref] [PubMed]
- Marshall RS, Festa JR, Cheung YK, Chen R, Pavol MA, Derdeyn CP, Clarke WR, Videen TO, Grubb RL, Adams HP, Powers WJ, Lazar RM. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology 2012;78:250-5. [Crossref] [PubMed]
- Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109-37. [Crossref] [PubMed]
- Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. [Crossref] [PubMed]
- Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20:828. [Crossref] [PubMed]
- Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158-9. [Crossref] [PubMed]
- Bruno A, Shah N, Lin C, Close B, Hess DC, Davis K, Baute V, Switzer JA, Waller JL, Nichols FT. Improving modified Rankin Scale assessment with a simplified questionnaire. Stroke 2010;41:1048-50. [Crossref] [PubMed]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DLAmerican Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49:e46-e110. [Crossref] [PubMed]
- Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003. [Crossref] [PubMed]
- Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, Gupta R, Kirshner H, Megerian JT, Lesko J, Pitzer P, Ramos J, Castonguay AC, Barnwell S, Smith WS, Gress DRVISSIT Trial Investigators. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240-8. [Crossref] [PubMed]
- Bambakidis NC, Chowdhry SA. Cerebral revascularization for ischemic disease in the 21st century. J Neurointerv Surg 2010;2:229-36. [Crossref] [PubMed]
- Sangha RS, Naidech AM, Corado C, Ansari SA, Prabhakaran S. Challenges in the Medical Management of Symptomatic Intracranial Stenosis in an Urban Setting. Stroke 2017;48:2158-63. [Crossref] [PubMed]
- Lemmens R, Hamilton SA, Liebeskind DS, Tomsick TA, Demchuk AM, Nogueira RG, Marks MP, Jahan R, Gralla J, Yoo AJ, Yeatts SD, Palesch YY, Saver JL, Pereira VM, Broderick JP, Albers GW, Lansberg MG. DEFUSE 2, IMS III, STAR, and SWIFT trialists; DEFUSE 2 IMS III STAR and SWIFT trialists. Effect of endovascular reperfusion in relation to site of arterial occlusion. Neurology 2016;86:762-70. [Crossref] [PubMed]
- Turhon M, Kang H, Liu J, Zhang Y, Zhang Y, Huang J, et al. In-Stent Stenosis After Pipeline Embolization Device in Intracranial Aneurysms: Incidence, Predictors, and Clinical Outcomes. Neurosurgery 2022;91:943-51. [Crossref] [PubMed]
- Gao F, Sun X, Guo X, Li D, Xu GD, Miao ZR. Endovascular Recanalization of Symptomatic Nonacute Intracranial Internal Carotid Artery Occlusion: Proposal of a New Angiographic Classification. AJNR Am J Neuroradiol 2021;42:299-305. [Crossref] [PubMed]
- Ma L, Liu YH, Feng H, Xu JC, Yan S, Han HJ, Huang HE, Fang C, Tan HQ. Endovascular recanalization for symptomatic subacute and chronic intracranial large artery occlusion of the anterior circulation: initial experience and technical considerations. Neuroradiology 2019;61:833-42. [Crossref] [PubMed]
- Lu CJ, Kao HL, Sun Y, Liu HM, Jeng JS, Yip PK. Postprocedural complications after angioplasty with stenting of the internal carotid artery. Cerebrovasc Dis 2003;16:308-10. [Crossref] [PubMed]
- Ouriel K, Shortell CK, Illig KA, Greenberg RK, Green RM. Intracerebral hemorrhage after carotid endarterectomy: incidence, contribution to neurologic morbidity, and predictive factors. J Vasc Surg 1999;29:82-7; discussion 87-9. [Crossref] [PubMed]
- Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol 2004;43:1596-601. [Crossref] [PubMed]
- Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, Song SS, Yu WWEAVE Trial Sites and Interventionalists. WEAVE Trial: Final Results in 152 On-Label Patients. Stroke 2019;50:889-94. [Crossref] [PubMed]
- Li W, Zhu W, Wang A, Zhang G, Zhang Y, Wang K, Zhang Y, Wang C, Zhang L, Zhao H, Wang P, Chen K, Liu J, Yang X. Effect of Adjusted Antiplatelet Therapy on Preventing Ischemic Events After Stenting for Intracranial Aneurysms. Stroke 2021;52:3815-25. [Crossref] [PubMed]
- Zhang Y, Sun Y, Li X, Liu T, Liu P, Wang H, Ding J, Miao ZR, Li G. Early versus delayed stenting for intracranial atherosclerotic artery stenosis with ischemic stroke. J Neurointerv Surg 2020;12:274-8. [Crossref] [PubMed]
- Rice CJ, Cho SM, Taqui A, Moore NZ, Witek AM, Bain MD, Uchino K. Early versus Delayed Extracranial-Intracranial Bypass Surgery in Symptomatic Atherosclerotic Occlusion. Neurosurgery 2019;85:656-63. [Crossref] [PubMed]
- Li G, Wang N, Li X, Ma N, Liu T, Sun Y, Liu P, Miao Z, Zhang Y. Balloon-Mounted versus Self-Expanding Stent Outcomes in Symptomatic Middle Cerebral Artery Stenosis Combined with Poor Collaterals in China: A Multicenter Registry Study. World Neurosurg 2019;124:e675-81. [Crossref] [PubMed]
- Yoon NK, Awad AW, Kalani MY, Taussky P, Park MS. Stent technology in ischemic stroke. Neurosurg Focus 2017;42:E11. [Crossref] [PubMed]
- Mugge L, Mansour TR, Krafcik B, Mazur T, Floyd-Bradstock T, Medhkour A. Immunological, vascular, metabolic, and autonomic changes seen with aging possible implications for poor outcomes in the elderly following decompressive hemicraniectomy for malignant MCA stroke: a critical review. J Neurosurg Sci 2019;63:411-24. [Crossref] [PubMed]
- Arac A, Blanchard V, Lee M, Steinberg GK. Assessment of outcome following decompressive craniectomy for malignant middle cerebral artery infarction in patients older than 60 years of age. Neurosurg Focus 2009;26:E3. [Crossref] [PubMed]


