
Bedside ultrasound in tigecycline-associated acute pancreatitis: a case description
Introduction
Tigecycline-induced acute pancreatitis is a rare yet serious complication that requires immediate intervention since and according to case reports, can result in death (1-3). Although pancreatitis is typically secondary to cholecystitis, a limited percentage of cases occur with drug-induced pancreatitis. The incidence of pancreatitis associated with tigecycline is low, as reported in the results of phase 3 and 4 studies, ranging from 0.24–1% (1). A retrospective analysis of 205 cases of tigecycline treated at Zhejiang Provincial People’s Hospital between January 2021 and 2022 revealed only 1 case of pancreatitis, representing an incidence of about 0.5%. However, as reported by Kroner et al., transplant recipients are at higher risk for viral- and drug-induced acute pancreatitis (4). Okon et al. reported 62 cases of acute pancreatitis induced by tigecycline over 14 years [1997–2010] in the adverse drug reaction database of the US Food and Drug Administration (2).
This case report describes a 62-year-old male who was comatose due to cerebral hemorrhage, which made identification of symptoms difficult and led to the possibility of misdiagnosis. Via clinical evidence and a literature review, we describe the clinical diagnosis of tigecycline-induced acute pancreatitis and recommend clinical countermeasures, which can serve as a reference to improve the understanding of this complication. Bedside ultrasound is an essential tool in the diagnosis of acute pancreatitis, enhancing the timeliness and sensitivity of diagnosis while minimizing misdiagnosis and missed diagnosis.
Case presentation
A 62-year-old man experienced a cerebral hemorrhage 1 year prior that left him comatose despite his having undergone craniotomy and hematoma removal. He was transferred to the rehabilitation department for further care. Although he had a history of hypertension and chronic renal insufficiency, he had no record of cholecystitis or pancreatitis. On admission, his bilirubin, alanine aminotransferase, and amylase levels were normal. Abdominal ultrasonography and computed tomography (CT) (Figure 1A) indicated fatty liver, normal gallbladder and pancreas, and no peritoneal effusion. During his hospitalization, he repeatedly developed aspiration pneumonia and hypostatic pneumonia. Due to a fever and lung infection, the prescribed treatment regimen of amikacin, levofloxacin, and meropenem proved ineffective. Sputum and blood cultures revealed the presence of Klebsiella pneumoniae. Since the patient was unresponsive to carbapenem antibiotics and the drug susceptibility test showed sensitivity to tigecycline, he was administered 100 mg of tigecycline every 12 hours intravenously for 5 days, followed by 50 mg every 12 hours, which led to significant improvement in his pulmonary infection. All procedures performed in this study were in accordance with the relevant ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

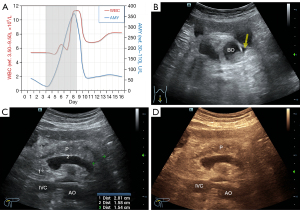
On the fifth day after receiving intravenous tigecycline, the comatose patient showed abdominal distension and dullness upon percussion. As the patient was unable to describe his symptoms independently, bedside ultrasound was conducted to rule out ascites. Abdominal CT examination was then performed (Figure 1B-1D). Bedside ultrasound results revealed diffuse pancreatic enlargement, with anteroposterior diameters of the head, body, and tail measuring 37, 24, and 26 mm (Figure 2A), respectively (normal values are <25, 20, and 20 mm, respectively). The local outline was unclear, and a small hypoechoic area was observed (indicating local edema or hemorrhage) (Figure 2B). In addition, a narrow liquid dark area was present in front of the pancreas (Figure 2B) while another dark area was noted in the abdominal cavity above the bladder (Figure 2C). Color Doppler flow imaging (CDFI) showed normal blood flow in the splenic vein without any apparent thrombus (Figure 2D). A blood amylase test revealed a sharp increase to 361 (normal range, 30–110) U/L, and the counts of white blood cells increased to 11.19 (normal range, 3.50–9.50) ×109/L. Subsequent abdominal CT showed pancreatic enlargement with inflammatory changes around the pancreas and peritoneal effusion (Figure 1B,1C), which confirmed the diagnosis of acute pancreatitis. According to the revised Atlanta classification and definitions from international consensus (5), these observations indicated acute pancreatitis, acute peripancreatic fluid collection (APFC), and moderate peritoneal effusion.

The patient was diagnosed with acute pancreatitis by gastroenteropancreatic surgery experts based on the examination results and clinical manifestations. The evidence chain supported the diagnosis of tigecycline-induced acute pancreatitis. Therefore, tigecycline was immediately discontinued, and the patient underwent a 2-day treatment regimen of fasting, gastrointestinal decompression, and combined anti-infection, acid suppression, and inhibition of pancreatic juice secretion. The patient’s abdominal distension improved, blood amylase levels dropped to 65 (normal range, 30–110) U/L (Figure 3A), and the white blood cell count recovered to 7.11 (normal range, 3.50–9.50) ×109/L (Figure 3A). Bedside ultrasound showed reduced anteroposterior diameters of the pancreas’ head, body, and tail (28, 16, and 15 mm respectively) with fuzzy edges. The overall echo was lower than previous, indicating an increased edema area in the pancreas, and the previously observed dark liquid area around the pancreas had disappeared. However, a small amount of dark liquid area was found in the intestinal space (yellow arrow in Figure 3B), indicating that the appearance of pancreatitis had improved since the prior examination (Figure 3B-3D). CT re-examination results (Figure 1D) showed a decrease in exudate around the pancreas and absorption of peritoneal effusion compared to the previous examination. The patient was discharged from the hospital at his family’s request due to no hope of waking and then died of complications caused by an unknown drug 2 months later.
Discussion
Acute pancreatitis is commonly caused by gallbladder stones and alcohol consumption, while other factors such as high triglycerides, hypercalcemia, viral infections, and certain drugs can also be the underlying causes. Tigecycline-induced acute pancreatitis is a rare occurrence, and the diagnosis in this case was made through ultrasonography, which provided clinical observation and diagnostic support. The purpose of this case report is to present the clinical features and imaging findings of this uncommon complication. The mechanism by which tigecycline induces pancreatitis is not yet fully understood, but it is believed to be similar to that of tetracycline antibiotics. Several possible mechanisms have been proposed based on various studies: (I) tetracyclines can produce toxic substances that induce pancreatitis (1); (II) the intestinal motility-promoting effect of tetracyclines stimulate the release of a large amount of motilin, leading to the spasm of Oddi’s sphincter and inducing pancreatitis (6); (III) tigecycline accumulates in large amounts in bile, which can lead to pancreatitis (7); (IV) tigecycline can inhibit the activity of cytochrome P4503A4 (CYP3A4), which hinders the metabolism of tigecycline in phase I, leading to the accumulation of the drug in the body (8); (V) in several reported cases, the patients had a history of renal insufficiency or renal transplantation (8,9). Since tigecycline is excreted through the kidneys, it may lead to increased bioaccumulation of the drug in the body, thus inducing pancreatitis. However, the specific reasons for this are still unclear and require further investigation.
Initial imaging assessment of patients presenting with gastric pain and suspected acute pancreatitis typically involves transabdominal ultrasonography (10). According to the current eligibility criteria established by the American College of Radiology (ACR), ultrasound is considered the “generally recommended” imaging modality for patients with suspected acute pancreatitis within the first 48–72 hours. However, it should be noted that ultrasound is not intended to replace CT or magnetic resonance imaging in cases of atypical pancreatitis, critically ill patients, or patients suspected of having complications related to acute pancreatitis. Consequently, in patients with well-defined clinical presentations and appropriate elevations of amylase and lipase, CT imaging may not yield additional diagnostic information. Ultrasound imaging is typically the primary choice for evaluating acute abdominal pain in most healthcare facilities due to its reproducibility and suitability to conducting bedside examinations (11).
When acute pancreatitis is suspected, dynamic contrast-enhanced CT is typically the preferred imaging modality. However, since only a quarter of patients with acute pancreatitis develop necrosis within the first 24–48 hours, it is recommended to delay CT scans until 72 hours after symptom onset (12). In such cases, ultrasound exploration during this window period may be considered. In this particular case, the patient was comatose and bedridden, so bedside ultrasound was used as a screening method. Ultrasonography plays a pivotal role in the diagnosis of acute pancreatitis and can reveal the following key diagnostic indicators: (I) pancreatic enlargement, which can manifest as either diffuse or localized enlargement; (II) hypoechoic or anechoic regions within the pancreas, which may involve the entire organ or specific segments; and (III) presence of inflammatory exudate surrounding the pancreas. Furthermore, ultrasonography enables the identification of common complications associated with acute pancreatitis, such as acute peripancreatic effusion, pancreatic pseudocyst, splenic vein thrombosis, pseudoaneurysm, and pleural effusion. Ultrasound can provide quantitative information on pancreas size, edema-related changes in internal echo, and the presence of exudate. It can also complement CT images by identifying vascular complications (13). Pancreatitis may be suspected or diagnosed via ultrasound (even bedside or better point-of-care ultrasound examination) when there are consistent findings such as pancreatic enlargement, blurred edges, peripancreatic fluid etc. The accuracy, convenience, and rationality of the initial ultrasound examination were confirmed in this case. Ultrasound can also guide diagnosis or intervention and may even be the only imaging modality for acute pancreatitis in children (13). In line with previous literature (14), the patient’s white blood cell count showed a transient increase, and the amylase index began to decline on the second day after drug withdrawal and symptomatic treatment, returning to normal on the third day. Unfortunately, the patient died of inexplicable drug complications after discharge, which has been reported in similar cases (3), highlighting the need for clinicians to remain vigilant for such complications.
Conclusions
When tigecycline is used, abdominal pain, distension, or unexplained increases in amylase should raise suspicion of acute pancreatitis induced by the drug. Routine monitoring of amylase and white blood cell counts during medication administration is necessary, particularly in cases of renal insufficiency or kidney transplantation. Bedside ultrasonography is an optimal imaging screening method for this complication due to its speed, high sensitivity, and lack of radiation.
Acknowledgments
We are grateful to Dr. Litao Sun from the Center for Reproductive Medicine, Department of Ultrasound Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College and Dr. Xiangming Ye from the Center for Rehabilitation Medicine, Rehabilitation & Sports Medicine Research Institute of Zhejiang Province, Department of Rehabilitation Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College for their guidance and advice during the implementation process of the study. Moreover, we would like thank Dr. Weidong Ge from the Cancer Center, Department of Ultrasound Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College for funding this study. We would also like to express our appreciation to all doctors and other hospital staff for their efforts in this study and to all patients for their participation.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-260/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGovern PC, Wible M, Korth-Bradley JM, Quintana A. Pancreatitis in tigecycline Phase 3 and 4 clinical studies. J Antimicrob Chemother 2014;69:773-8. [Crossref] [PubMed]
- Okon E, Engell C, van Manen R, Brown J. Tigecycline-related pancreatitis: a review of spontaneous adverse event reports. Pharmacotherapy 2013;33:63-8. [Crossref] [PubMed]
- Wang PF, Zou H, Zhu JH, Shi FE. Acute pancreatitis caused by tigecycline: A case report and literature review. Medicine (Baltimore) 2021;100:e28245. [Crossref] [PubMed]
- Kroner PT, Mareth K, Raimondo M, Lee DD, Alsaad A, Aslam N, Abader P, Wadei HM. Acute Pancreatitis in Advanced Chronic Kidney Disease and Kidney Transplant Recipients: Results of a US Nationwide Analysis. Mayo Clin Proc Innov Qual Outcomes 2019;3:160-8. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SSAcute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol 2007;5:648-61; quiz 644. [Crossref] [PubMed]
- Greer ND. Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent) 2006;19:155-61. [Crossref] [PubMed]
- Pavan M, Chaudhari AP, Ranganth R. Altered bioavailability of tacrolimus following intravenous administration of tigecycline. Am J Kidney Dis 2011;57:354. [Crossref] [PubMed]
- Lin J, Wang R, Chen J. Tigecycline-induced acute pancreatitis in a renal transplant patient: a case report and literature review. BMC Infect Dis 2018;18:201. [Crossref] [PubMed]
- Burrowes DP, Choi HH, Rodgers SK, Fetzer DT, Kamaya A. Utility of ultrasound in acute pancreatitis. Abdom Radiol (NY) 2020;45:1253-64. [Crossref] [PubMed]
- Porter KK, Zaheer A, Kamel IR, Horowitz JM, Arif-Tiwari H, Bartel TB, Bashir MR, Camacho MA, Cash BD, Chernyak V, Goldstein A, Grajo JR, Gupta S, Hindman NM, Kamaya A, McNamara MM, Carucci LR. ACR Appropriateness Criteria® Acute Pancreatitis. J Am Coll Radiol 2019;16:S316-30. [Crossref] [PubMed]
- Shah AP, Mourad MM, Bramhall SR. Acute pancreatitis: current perspectives on diagnosis and management. J Inflamm Res 2018;11:77-85. [Crossref] [PubMed]
- Zhou XL, Baikeli D, Yin DF. Literature Analysis of 11 Cases of Tigecycline-induced Acute Pancreatitis in Renal Transplant Patients. Chinese Journal of Pharmacoepidemiology 2022;31:492-6.
- Li X, Li L, Liu T, Hai X, Sun B. Leukocytosis induced by tigecycline in two patients with severe acute pancreatitis. Br J Biomed Sci 2021;78:225-8. [Crossref] [PubMed]


