
Noninvasive transthoracic doppler flow velocity and invasive thermodilution to assess coronary flow reserve
Introduction
The coronary flow reserve (CFR) is a well-validated index that can be used to assess coronary circulation and functional impairment. The CFR also provides prognostication in various patient subsets (1). CFR reflects the integrated coronary disease burden, including epicardial functional stenosis severity and microvascular dysfunction (1,2). Given the limited availability of pressure-velocity wires such as ComboWire (Phillips Volcano, San Diego, California, USA), thermodilution-derived coronary flow reserve (CFRthermo) has become an increasingly used invasive method to measure CFR and evaluate coronary circulation in catheter laboratories because of its wide availability and ease of performance. Stress transthoracic Doppler echocardiography (S-TDE) of the left anterior descending artery (LAD) is a non-invasive method for assessing CFR based on flow velocity. CFRS-TDE is a low-cost, widely available, and efficiently performed method that does not require radiation or contrast, and the results have good agreement with intracoronary Doppler wire-derived CFR and positron emission tomography (PET)-derived myocardial flow reserve (3-7). However, data on the association between CFRS-TDE and CFRthermo are currently limited. CFR, similar to the microcirculatory resistance (IMR) index, may reflect microvascular function after the successful modification of functionally significant epicardial stenosis by percutaneous coronary intervention (PCI). The aim of this study was to compare CFRS-TDE and CFRthermo in the LAD with functionally significant proximal lesions before and after PCI. Further, we evaluated the correlation of IMR with CFRS-TDE and CFRthermo before and after PCI.
Methods
Study design and patient population
This is a prospective study including patients with chronic coronary syndrome (CCS) who were planned to undergo elective PCI for de novo, single, hemodynamically significant lesions in proximal LAD at Tsuchiura Kyodo General Hospital, a single tertiary-care hospital in Japan between April 1, 2019 and November 30, 2022. All patients suffered from anginal symptoms (Canadian Cardiovascular Society class 1–3) and LAD lesions with fractional flow reserve (FFR) ≤0.80. Eligible patients were enrolled from our regular clinical population. The exclusion criteria were (I) acute coronary syndrome or total occlusion, (II) LAD lesions exhibiting angiographically visible collateral flow, (III) inability to provide consent to the study protocol, (IV) previous LAD PCI or myocardial infarction, (V) coronary artery bypass graft surgery, (VI) left main significant disease, (VII) chronic renal disease having baseline serum creatinine level >1.5 mg/dL, (VIII) decompensated heart failure, (IX) cardiomyopathy, (X) atrial fibrillation, and (XI) contraindications to adenosine administration. The patient selection flowchart is shown in Figure 1.

Guideline-directed medical therapy including high-dose statins, dual antiplatelets, β-blockers, and antihypertensives was started before or immediately after diagnostic angiography in all the patients. In accord with the study protocol, ad hoc PCI was not performed. After PCI, we further excluded patients with periprocedural myocardial infarction [as defined by the Fourth Universal Definition of Myocardial Infarction (8) based on a blood sample collected on average of 20–24 hours post PCI], ischemic symptoms, electrocardiogram (ECG) findings, and other newly detected imaging abnormalities after PCI completion because it had been reported that such events affect post-PCI physiological parameters (9). In the final analysis, we included the patients with minor cardiac troponin elevation without other manifestations required by the abovementioned definition.
This study was approved by the Institutional Ethics Committee of Tsuchiura Kyodo General Hospital (approval No. 809) and was performed in compliance with the tenets of the Declaration of Helsinki (as revised in 2013) for human studies. Written informed consent for the study and future anonymous data utilization was taken from all the patients.
Invasive diagnostic coronary angiography
Each patient initially underwent standard diagnostic coronary angiography via the radial artery using a 5F system for assessment of the coronary anatomy and functional stenosis severity by FFR measurements. The CMS-MEDIS system (Medis Medical Imaging Systems, Leiden, The Netherlands) was used for quantitative coronary angiography analyses. All patients received a bolus injection of heparin (5,000 IU) before the procedure. We administered intracoronary bolus injections of nitroglycerin (0.2 mg) at the beginning of the procedure and before functional assessments. Patients with LAD lesions showing FFR ≤0.80 were eligible and subsequently enrolled.
PCI and physiological assessments
All patients underwent FFR-guided PCI according to guidelines (10,11). Pre- and post-PCI physiological assessments were performed with a temperature-sensitive pressure sensor-equipped guidewire (Abbott Vascular, St. Paul, MN, USA) using routine techniques (12,13). Briefly, a bolus injection of intracoronary nitroglycerin (0.2 mg) was administered, and then the wire sensor was advanced and positioned in the far distant of the target vessel. FFR value was defined as the ratio of the mean distal coronary pressure to the mean aortic pressure during stable maximum hyperemia, which was induced by the intravenous adenosine administration (140 µg/kg/min through a central venous route). After the FFR measurement, when the sensor window reached the tip of the guiding catheter during hyperemia via a pull-back maneuver, a mean pressure drift of ≤2 mmHg was confirmed. A repeat assessment was performed when the pressure drift was >2 mmHg, as mandated by institutional standard protocol. All patients were ensured that they had not consumed caffeine-containing beverages for at least 24 hours prior to catheterization. CFR was calculated as the ratio of the resting mean transit time (Tmn) to the hyperemic Tmn using a thermodilution technique. The IMR was calculated as the distal coronary pressure at maximum hyperemia divided by the inverse of the hyperemic Tmn. Post-PCI physiological assessments were performed in a similar way after post-stenting high-pressure dilatation with a noncompliant balloon and/or additional stenting guided by physiology and intracoronary imaging at the operators’ discretion and when the operator considered the final PCI procedure.
PCI techniques and the use of imaging devices, such as intravascular ultrasound or optical coherence tomography, and type of stent (drug-eluting stent) were left to the discretion of the interventionalists.
Measurement of coronary flow velocity using stress TDE
We performed coronary flow measurements in LAD using S-TDE before (1 day) and after (3 days) PCI for all eligible patients. Echocardiographic studies were conducted based on the guideline from the American Society of Echocardiography, using a commercially available digital ultrasound system (GE Vivid E95; GE Vingmed Ultrasound, Horten, Norway) with a multifrequency transducer and second-harmonic technology (12). Briefly, after the standard examination, coronary flow in the mid-distal portion of the LAD was evaluated in a modified 3-chamber view. For the color flow mapping, the velocity range was set to 16–24 cm/s. A sample volume (3–5 mm wide) was pointed at the distal LAD segment to measure coronary flow velocity. Peak diastolic coronary flow velocity was measured under basal resting conditions (bDPV) and during maximum hyperemia (hDPV) induced by intravenous adenosine administration (140 µg/kg/min through a central venous route). All the data were digitally stored for repeated offline reviews and analyses. Three appropriate flow signal profiles at rest and during hyperemia were collected from the recorded data.
The CFRS-TDE was obtained as the ratio of the hDPV to the bDPV using the software package of the ultrasound system. All stored data were separately analyzed twice at 1-week intervals by two experts, who were blinded to the clinical data, in order to evaluate the intra-observer reproducibility of the TTE-derived data. To evaluate the effect of measurement variability on the measurement of diastolic peak velocity by S-TDE, two independent observers (YH and TN), who were blinded to the patients’ physiological results, analyzed 50 randomly selected Doppler velocity tracing records. Each observer had no access to the readings of the other observer or CFRthermo data. Inter-observer variability was evaluated as the standard deviation of the differences between the two observers, defined as a percentage of the average value. Intra-observer reproducibility was repeated by one observer 5 min apart in 30 patients who underwent S-TDE measurements twice. All patients were instructed to avoid caffeine consumption for at least 24 hours before S-TDE. Figure 2 shows a representative case of the CFRS-TDE recording and measurements in the LAD before and after LAD PCI.

Statistical analysis
Categorical data were expressed as the number and percentage and compared using the chi-square or Fisher’s exact test, as appropriate. The normality of the distributed values was assessed using Shapiro-Wilk tests. Continuous variables were showed as the mean ± standard deviation for normally distributed variables or as the median (25th–75th percentile) for non-normally distributed variables and were compared using Student’s t-tests and the Mann-Whitney U-test, respectively. Associations were evaluated by analyzing Pearson’s correlation for normally distributed data and Spearman’s correlation for non-normally distributed data. Bland-Altman analyses were used to test for agreement. The kappa statistic was used to test the inter-rater reliability of CFR values before and after PCI. Receiver-operating characteristic (ROC) curves were analyzed to assess the best cutoff value of pre-PCI CFRthermo values corresponding to the CFRS-TDE cutoff value. The optimal cutoff value was determined using the Youden index. All statistical analyses were performed using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P<0.05.
Results
Baseline patient characteristics and angiographical and physiological findings
Among the 205 patients who were initially enrolled and underwent PCI, 24 patients were excluded due to incomplete S-TDE data acquisition (n=15), incomplete thermodilution data (n=7), termination of S-TDE testing because of the appearance of atrioventricular block (n=1), and withdrawal of consent (n=1). Additionally, 7 patients who showed type 4A myocardial infarction were also excluded from the final analysis. Finally, 174 patients who had successful FFR-guided LAD PCI with complete pre- and post-PCI S-TDE and wire-based physiological data were included in the analysis. Tables 1,2 summarize the characteristics of the 174 patients. Tables 3,4 show the pre- and post-PCI angiographic and physiological data respectively. The median pre-PCI FFR, CFRthermo, CFRS-TDE were 0.70, 2.05, 1.89, respectively, while the median post-PCI FFR, CFRthermo, and CFRS-TDE were 0.83, 2.59, and 2.33, respectively. In all cases, FFR significantly improved after PCI, from 0.70 (0.62–0.74) before PCI to 0.83 (0.80–0.87) after PCI (P<0.001). Both CFRthermo and CFRS-TDE were also significantly increased after PCI (P<0.001 and P<0.001 respectively, Tables 3,4), although the CFRthermo and CFRS-TDE are decreased in 42% and 23% of the patients, respectively. The hyperemic diastolic peak velocity detected by S-TDE significantly increased from 51 to 69 cm/s (P<0.001). CFRS-TDE similarly increased after PCI, although 41 (23%) patients showed a decrease in post-PCI CFRS-TDE. Both CFRthermo and CFRS-TDE showed a modest correlation with the pre-PCI FFR (r=0.383 and r=0.366, respectively; Figure S1A,S1B).
Table 1
| Parameter | Total (n=174) |
|---|---|
| Age, years | 68 (60–77) |
| Male | 132 (75.9%) |
| Hypertension | 143 (82.1%) |
| Diabetes mellitus | 81 (46.6%) |
| Dyslipidemia | 97 (55.7%) |
| Smoker | 111 (63.8%) |
| eGFR (mL/min/1.73 m2) | 64.6 (50.7–75.2) |
| LDL-cho (mg/dL) | 78 (65–103) |
| NT-proBNP (pg/mL) | 209 (72–740) |
| HbA1c (%) | 6.2 (5.8–6.9) |
| hs-troponin I (ng/L) | 11 (5–41) |
| QCA analysis | |
| QCA MLD | 1.11 (0.82–1.39) |
| QCA RD | 2.49 (2.14–2.91) |
| QCA %stenosis | 54.7 (43.4–64.8) |
| QCA lesion length | 22.5 (15.3–33.1) |
Values are presented as n (%), or median (interquartile range). eGFR, estimated glomerular filtration rate; LDL-cho, low density lipoprotein cholesterol; NT-proBNP, N‑terminal pro-brain natriuretic peptide; HbA1c, Hemoglobin A1c; hs-troponin, high sense-troponin I; QCA, quantitative coronary angiography; MLD, minimal lumen diameter; RD, reference lumen diameter; %stenosis, percent diameter stenosis.
Table 2
| Parameter | Total (n=174) |
|---|---|
| LVDd (mm) | 46 [42–50] |
| LVDs (mm) | 29 [26–34] |
| IVS (mm) | 12 [10–13] |
| PW (mm) | 12 [10–13] |
| LVEF (%) | 64 [54–69] |
| LA diameter (mm) | 39 [35–42] |
| E/A ratio | 0.8 [0.6–1.0] |
Values are presented as median [interquartile range]. LVDd, left ventricular end-diastolic diameter; LVDs left ventricular end-systolic diameter; IVS, interventricular septum thickness; PW posterior wall thickness; LVEF, left ventricular ejection fraction; LA diameter, left atrial diameter.
Table 3
| Parameter | Total (n=174) |
|---|---|
| FFR | 0.70 (0.62–0.74) |
| CFRthermo | 2.05 (1.38–2.93) |
| CFRS-TDE | 1.89 (1.44–2.31) |
| IMR | 23.4 (18.2–34.6) |
| Base Tmn (s) | 0.97 (0.68–1.31) |
| Hyper Tmn (s) | 0.44 (0.32–0.63) |
| Base DPV (cm/s) | 26.0 (20.5–32.5) |
| Hyper DPV (cm/s) | 51.0 (38.0–66.5) |
Values are presented as median (interquartile range). PCI, percutaneous coronary intervention; FFR, functional flow reserve; CFRthermo, thermodilution-derived coronary flow reserve; CFRS-TDE, stress transthoracic Doppler echocardiography-derived coronary flow velocity reserve; IMR index of microcirculatory resistance; Tmn, mean transit time; DPV, diastolic peak velocity.
Table 4
| Parameter | Total (n=174) |
|---|---|
| FFR | 0.83 (0.80–0.87) |
| CFRthermo | 2.59 (1.63–3.55) |
| CFRS-TDE | 2.33 (1.91–2.90) |
| IMR | 21.0 (15.5–30.4) |
| Base Tmn (s) | 0.89 (0.53–1.20) |
| Hyper Tmn (s) | 0.32 (0.23–0.45) |
| Base DPV (cm/s) | 29.0 (24.0–35.0) |
| Hyper DPV (cm/s) | 69.0 (53.5–84.0) |
Values are presented as median (interquartile range). PCI, percutaneous coronary intervention; FFR, functional flow reserve; CFRthermo, thermodilution-derived coronary flow reserve; CFRS-TDE, stress transthoracic Doppler echocardiography-derived coronary flow velocity reserve; IMR index of microcirculatory resistance; Tmn, mean transit time; DPV, diastolic peak velocity.
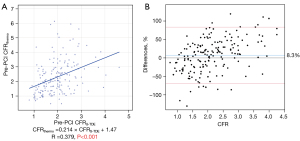
Agreement between pre-PCI CFRthermo and CFRS-TDE
The median pre-PCI CFRthermo was significantly higher than the pre-PCI CFRS-TDE [2.05 (1.38–2.93) vs. 1.89 (1.44–2.31), P<0.001]. The commonly used CFRS-TDE cutoff value of 2.0 corresponded to 2.18 of CFRthermo as determined by ROC analysis [AUC: 0.710 (0.633–0.787)]. Pre-PCI CFRthermo and CFRS-TDE had a moderate correlation (r=0.379, P<0.001, Figure 3A). The corresponding Bland-Altman plot indicated a bias of 8.3% (Figure 3B), and the intraclass correlation coefficient was 0.534 (P<0.001). The concordance rate for diagnosing decreased pre-PCI CFR using a cutoff value of 2.0 for CFRS-TDE and the corresponding CFRthermo of 2.18 obtained by ROC analysis was 0.317 by kappa value.
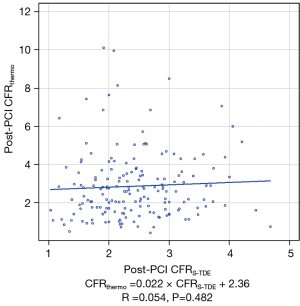
Agreement between post-PCI CFRthermo and CFRS-TDE
The median (interquartile range) post-PCI CFRthermo and CFRS-TDE values were 2.59 (1.63–3.55) and 2.33 (1.91–2.90), respectively. Notably, no significant correlation was found between post-PCI CFR values obtained by two methods (Figure 4). Furthermore, no significant correlation was detected between delta CFRthermo and delta CFRS-TDE (r=0.008, P=0.915). After PCI, the concordance rate of diagnosing decreased post-PCI CFR using 2.0 as the unified cutoff value for both CFRS-TDE and CFRthermo was 0.078 by kappa value.
Relationship between microvascular function and CFR
There was a modest correlation between pre-PCI CFRthermo and pre-PCI IMR (r=−0.393, P<0.001), while a very weak but significant relationship was found between pre-PCI CFRS-TDE and pre-PCI IMR (r=−0.155, P=0.041). Post-PCI IMR showed a statistically significant moderate correlation with post-PCI CFRthermo (r=−0.343, P<0.001), whereas no significant correlation was found between post-PCI IMR and post-PCI CFRS-TDE (r=−0.109, P=0.151). Association between IMR and CFRthermo, IMR and CFRS-TDE are shown in Figure S2.
Interobserver variability and reproducibility of S-TDE measurements
Good agreement was observed between the two independent observers’ measurements of diastolic peak velocity (r=0.92). The interobserver variability for diastolic peak velocity was 3.8%, and the intraclass correlation coefficient was 93% for accredited readers. The reproducibility of diastolic peak velocity was 3.2% between first and second (5 min apart) S-TDE measurements.
Discussion
The association between CFRS-TDE and CFRthermo has not been clarified yet. The main findings of this study are as follows. First, there is a moderate correlation between CFRthermo and CFRS-TDE before PCI, while they are not associated after PCI. Second, both CFRthermo and CFRS-TDE are increased after PCI in a total cohort, although the CFRthermo and CFRS-TDE are decreased in 42% and 23% of the patients, respectively. Third, the CFRthermo is significantly higher than CFRS-TDE both pre- and post-PCI. Fourth, the pre-PCI CFRthermo has a modest correlation with pre-PCI IMR, while pre-PCI CFRS-TDE has a very weak but borderline significant relationship with pre-PCI IMR. Fifth, post-PCI IMR has a significant correlation with post-PCI CFRthermo, while there is no significant correlation between post-PCI IMR and post-PCI CFRS-TDE. Sixth, the concordant rate of diagnosing decreased CFR using a cutoff value of 2.0 for pre-PCI CFRS-TDE and the corresponding pre-PCI CFRthermo of 2.18 is 0.317 by kappa value and 0.078 for post-PCI CFR when applying the unified cutoff value of 2.0. To our best knowledge, the present study is the first to compare non-invasive transthoracic Doppler echocardiography (TDE)-derived flow velocity and invasive thermodilution-derived physiological measures for the assessment of pre- and post-PCI CFR before and after elective PCI, although the examination time window was different.
Correlation between pre-PCI CFRS-TDE and pre-PCI CFRthermo measurements
Previous studies reported that noninvasive measurements of coronary flow velocity and CFR by S-TDE in the LAD accurately reflected the invasive measurement of coronary flow velocity and CFR using a Doppler flow velocity wire and the noninvasive standard reference method by PET (4,5). Our results are consistent with those of Demir et al. (7) who demonstrated that CFRthermo overestimated invasively measured velocity-derived CFR, while both parameters showed a modest correlation before PCI. The observed systemic bias was not uniform from low to high CFR values. Further, compared to CFRS-TDE, CFRthermo introduced higher overestimation, as indicated by higher CFR values (Figure 3B). Our results are consistent with previous studies comparing CFRthermo with invasive velocity wire-derived CFR values. However, to our best knowledge, we are the first to use noninvasive S-TDE data for comparison with CFRthermo in patients treated with PCI (7,13). In contrast to CFRthermo, S-TDE-derived or invasive wire-derived CFR provides theoretically and clinically more robust reference markers for coronary flow volume, as reported in previous studies (4,5,13). Notably, invasive wire-derived Doppler flow velocity method is challenging for obtaining optimal signal data (14), whereas CFRS-TDE is widely available and has good reproducibility with a high level of sufficient signal tracing, as shown in this study (190/205, 92.7%). Considering these circumstances, CFRS-TDE may be more feasible and robust than invasive Doppler wire-derived CFR and CFRthermo as routine assessments of CFR in clinical practice.
The exact mechanism of the overestimation of CFRthermo remains undetermined. When using CFR for the assessment of coronary physiology, differences in the cutoff values for stratification or diagnosis according to the methodology of measuring CFR values should be noted.
Correlation between post-PCI CFRS-TDE and post-PCI CFRthermo measurements
In contrast to the results of the pre-PCI comparison of CFRS-TDE and CFRthermo, post-PCI CFRthermo showed no significant correlation with CFRS-TDE, which has not been reported previously. Notably, the discordant rate between post-PCI CFRthermo and post-PCI CFRS-TDE was 41%, using 2.0 as the cutoff value for both post-PCI CFRS-TDE and post-PCI CFRthermo. This could be explained as follows. First, the measurement window for CFR from the completion of PCI by each method was different [CFRthermo, immediately after PCI completion; CFRS-TDE, 3 days (median) after PCI completion]. Compared with CFRthermo, CFRS-TDE is more likely to represent stable post-PCI coronary circulation (15,16). Second, post-PCI variability of transit time or inconsistent surrogate marker of coronary flow by transit time compared with S-TDE derived flow velocity (7). Further studies are needed to elucidate the clinical significance of the differences between these two post-PCI CFR metrics and their clinical relevance. The invasive CFR measurement by wire-derived flow velocity depends on the technical expertise of the operator, and suboptimal signal quality of Doppler traces has been reported to occur in as high as 30% of measurements (14). In contrast, both CFRthermo and noninvasive CFRS-TDE provide very high level of signal tracing performance for obtaining CFR (198/205, 96.6% and 190/205, 92.7%, respectively in this study). It is unlikely that the population bias might have caused the discordant results between these two post-PCI metrics in the present study because the subjects were prospectively enrolled following an all-comers design according to the study protocol in routine clinical practice. The relationships between pre- and post-PCI inverse of resting and hyperemic Tmn and TDE-derived velocity, both proposed as surrogate markers of coronary flow, is shown in Figure S3. In contrast to the pre-PCI relationship, no significant relationship was found between post-PCI hyperemic flow velocity and post-PCI inverse of hyperemic Tmn. This observation may at least partially explain the discordant results of the CFR values between the two methods after PCI.
Microvascular resistance by IMR or CFR after PCI
Microvascular dysfunction is increasingly noted to be a cause of myocardial ischemia (17,18). Coronary microvascular disease and its significance in cardiac events among patients without significant obstructive disease have also been reported (1,19). Recent studies have reported the coexistence of coronary microvascular dysfunction and epicardial obstructive disease (20,21). In the present study, residual microvascular dysfunction after epicardial stenosis removal by PCI may be relevant to myocardial ischemia or cardiac events possibly caused by microvascular dysfunction after PCI. In the absence of obstructive epicardial disease, the current ESC guidelines recommend the measurement of CFR or IMR for the assessment of microvascular function in clinical practice, without referring to which modality is the best for the CFR measurements (22). Given that no significant relationship was observed between post-PCI CFRS-TDE and IMR or between post-PCI CFRS-TDE and CFRthermo, the modality to obtain or the metric to represent post-PCI microvascular function should be chosen or interpreted considering the results of this study. Our results are consistent with those of a previous report showing no correlation between thermodilution-derived IMR and Doppler-derived microvascular resistance (7). A clinically applicable reference measure of microvascular resistance remains inconclusive, limiting the ability to compare the diagnostic accuracy of these two indices. Further studies are needed to elucidate which index accurately reflects microvascular function after PCI or if the combination of these metrics could predict worse future outcomes.
Limitations
This was a single-center retrospective analysis of prospectively registered patient data and relevant to an observational nature; thus, inherent limitations exist. The number of study patients was limited by rigorous inclusion and exclusion criteria, possibly leading to a certain level of selection bias, although all patients were prospectively included in routine clinical practice. The present study was conducted in a group of patients with epicardial functional stenosis and future studies are needed to investigate the relationship between CFRS-TDE and CFRthermo and their prognostic efficacy in non-flow-limiting lesions or patients with ischemia and non-obstructive coronary artery. In the Doppler echocardiographic interrogation of coronary flow in the LAD, LAD flow data before and after PCI were comparable only at identical guidewire positions and fixed vessel diameters under similar hemodynamic conditions. However, a change in the LAD diameter after PCI or a difference in the guidewire position cannot be ruled out. The measurement window of CFR by each approach was different (CFRthermo, immediately after PCI completion; CFRS-TDE, 3 days after PCI completion) and this difference could have affected the results. The absolute coronary flow volume, such as in PET studies as a reference, was not assessed in this study. Importantly, hyperemic conditions in the present study were achieved by intravenous infusion of adenosine, indicating the hyperemia could be induced in pancoronary territories. Given microvascular function is affected by collateral flows from non-LAD territories, intracoronary adenosine infusion might better induce hyperemia only in the LAD territory. Finally, this study used the thermodilution method for the CFRthermo and IMR. Currently, neither RayFlow® catheters (HEXACATH, Paris, France) nor a 0.014” dual pressure and Doppler velocity sensor-tipped guidewire (ComboWire XT, Philips Volcano, San Diego, CA, USA) are commercially accessible in Japan. These methods may provide different wire-based CFR and hyperemic absolute microvascular resistance when commercially available worldwide, thus further investigations are warranted.
Conclusions
Pre-PCI CFRthermo and CFRS-TDE have modest correlation, whereas post-PCI CFRthermo and CFRS-TDE have no significant correlation. There is a modest correlation between CFRthermo and IMR before and after PCI, whereas there is no significant correlation between post-PCI CFRS-TDE and post-PCI IMR. CFRS-TDE and CFRthermo are not interchangeable, particularly post-PCI. This suggests that the two metrics represent different post-PCI coronary physiology.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-416/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Ethics Committee of Tsuchiura Kyodo General Hospital (approval No. 809) and was performed in compliance with the tenets of the Declaration of Helsinki (as revised in 2013) for human studies. Written informed consent for the study and future anonymous data utilization was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelshiker MA, Seligman H, Howard JP, Rahman H, Foley M, Nowbar AN, Rajkumar CA, Shun-Shin MJ, Ahmad Y, Sen S, Al-Lamee R, Petraco R. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J 2022;43:1582-93. [Crossref] [PubMed]
- van de Hoef TP, Siebes M, Spaan JA, Piek JJ. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J 2015;36:3312-9a. [Crossref] [PubMed]
- Caiati C, Montaldo C, Zedda N, Montisci R, Ruscazio M, Lai G, Cadeddu M, Meloni L, Iliceto S. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol 1999;34:1193-200. [Crossref] [PubMed]
- Saraste M, Koskenvuo J, Knuuti J, Toikka J, Laine H, Niemi P, Sakuma H, Hartiala J. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol 2001;21:114-22. [Crossref] [PubMed]
- Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol 1998;32:1251-9. [Crossref] [PubMed]
- Olsen RH, Pedersen LR, Snoer M, Christensen TE, Ghotbi AA, Hasbak P, Kjaer A, Haugaard SB, Prescott E. Coronary flow velocity reserve by echocardiography: feasibility, reproducibility and agreement with PET in overweight and obese patients with stable and revascularized coronary artery disease. Cardiovasc Ultrasound 2016;14:22. [Crossref] [PubMed]
- Demir OM, Boerhout CKM, de Waard GA, van de Hoef TP, Patel N, Beijk MAM, Williams R, Rahman H, Everaars H, Kharbanda RK, Knaapen P, van Royen N, Piek JJ, Perera D. Comparison of Doppler Flow Velocity and Thermodilution Derived Indexes of Coronary Physiology. JACC Cardiovasc Interv 2022;15:1060-70. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231-64. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44-122. [Crossref] [PubMed]
- Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e21-e129. [Crossref] [PubMed]
- Hildick-Smith DJ, Maryan R, Shapiro LM. Assessment of coronary flow reserve by adenosine transthoracic echocardiography: validation with intracoronary Doppler. J Am Soc Echocardiogr 2002;15:984-90. [Crossref] [PubMed]
- Everaars H, de Waard GA, Driessen RS, Danad I, van de Ven PM, Raijmakers PG, Lammertsma AA, van Rossum AC, Knaapen P, van Royen N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve: A Head-to-Head Comparison With [15O]H2O PET. JACC Cardiovasc Interv 2018;11:2044-54.
- Barbato E, Aarnoudse W, Aengevaeren WR, Werner G, Klauss V, Bojara W, Herzfeld I, Oldroyd KG, Pijls NH, De Bruyne B. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur Heart J 2004;25:219-23. [Crossref] [PubMed]
- Kawase Y, Omori H, Kawasaki M, Tanigaki T, Hirata T, Okamoto S, Ota H, Kikuchi J, Okubo M, Kamiya H, Hirakawa A, Suzuki T, Matsuo H. Postocclusional Hyperemia for Fractional Flow Reserve After Percutaneous Coronary Intervention. Circ Cardiovasc Interv 2017;10:e005674. [Crossref] [PubMed]
- Dupouy P, Aptecar E, Pelle G, Boudali L, Teiger E, Lanoue I, Veyssière F, Garot P, Pernès JM, Hovasse T, Kern MJ, Randé JL. Early changes in coronary flow physiology after balloon angioplasty or stenting: a 24-hour Doppler flow velocity study. Catheter Cardiovasc Interv 2002;57:191-8. [Crossref] [PubMed]
- Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1352-71. [Crossref] [PubMed]
- Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116:856-70. [Crossref] [PubMed]
- Mohammed AA, Zhang H, Abdu FA, Liu L, Singh S, Lv X, Shi T, Mareai RM, Mohammed AQ, Yin G, Zhang W, Xu Y, Che W. Effect of nonobstructive coronary stenosis on coronary microvascular dysfunction and long-term outcomes in patients with INOCA. Clin Cardiol 2023;46:204-13. [Crossref] [PubMed]
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830-40. [Crossref] [PubMed]
- Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:2625-41. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]


