
Hepatic anastomosing hemangioma: description of a rare case and a literature analysis
Introduction
Anastomosing hemangioma (AH), a tumor that was initially described by Montgomery and Epstein in 2009, is an extremely rare benign vascular. It is characterized by a unique histopathological architecture comprising anastomosing small-caliber capillaries and frequent hobnail endothelial cells (1). AHs show marked tendency to involve the genitourinary system and retroperitoneum, particularly the kidneys (2), with the liver being an uncommon site for AHs. Despite the benign nature of the tumors, the rarity of hepatic AHs and unfamiliarity with their imaging and pathological characteristics often lead to complications in accurate diagnosis and result in overtreatment.
We here present a case of AH in the liver which exhibited lesion growth during follow-up and which was ultimately diagnosed via surgical resection. A literature review of this uncommon hepatic lesion is further provided.
Case presentation
All procedures performed in this study were in accordance with the relevant ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
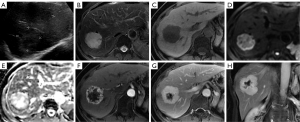
A 50-year-old woman was observed to have a lesion measuring 25 mm × 20 mm in the liver on ultrasound during routine physical examination. The initial diagnosis was hemangioma. She did not undergo regular surveillance. Seven years later, she visited our hospital because of intermittent, mild epigastric pain. Abdominal ultrasound revealed a well-defined, mixed-echoic mass with dotted blood flow signals in the right lobe of liver, measuring 50 mm × 47 mm (Figure 1A). Magnetic resonance imaging (MRI) showed a well-demarcated, lobulated mass measuring 52 mm × 51 mm × 45 mm in size. It appeared hypointense on T1-weighted imaging (T1WI) and hyperintense on T2-weighted imaging (T2WI), with no restricted diffusion and a mean apparent diffusion coefficient (ADC) value of 2.20×10−3 mm2/s (b=0,800). On enhanced MRI, the mass showed irregular, continuous rim-like enhancement in the early arterial phase that filled centripetally in the portal venous and equilibrium phases, with a persistent defect in the center (Figure 1B-1H). A tortuous feeding vessel was found on the arterial phase images. Her progastrin-releasing peptide (PROGRP) was slightly elevated at 82.8 pg/mL (normal range 25.0–78.0 pg/mL), while other laboratory tests (including routine blood test and liver function) were negative. She denied a history of hepatitis or malignancies. The diagnosis was indeterminate but was mostly consistent with a vascular tumor. Finally, the patient underwent laparoscopic tumor resection.
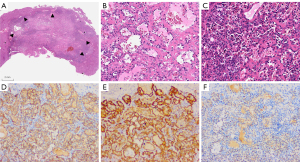
Histologically, the lesion appeared as a lobulated, well-defined mass without a capsule (Figure 2A). It exhibited a unique sinusoidal architecture composed of anastomosing small-caliber capillaries. The vascular structures were lined by a single layer of endothelial cells that frequently showed nuclear hobnailing (Figure 2B), and there was no obvious mitotic activity. Thrombosis, extramedullary hemopoiesis, and fibrous stroma were observed (Figure 2C). The immunohistochemistry results showed CD31+, CD34+, ERG+, and CK Pan– (Figure 2D-2F). The pathological diagnosis was AH. There was no evidence of tumor recurrence at 6-month postoperative follow-up.
Discussion
This report describes a case of hepatic AH with fairly comprehensive clinical, radiological, and pathological data. AH is a rare benign vascular tumor, and it is exceedingly uncommon for this entity to occur in the liver. Currently, only 23 cases of hepatic AH have been reported in the English-language literature (2-14) (Table 1), and thus our paper holds considerable significance for the future collection of imaging features and understanding of the natural course of this tumor.
Table 1
| Case | Author, year | Age (years) | Gender | History | Initial size (cm) | Lesion growth | Treatment | Follow-up (months) | Imaging features | Additional pathological or genetic findings |
|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 | Lin, 2013 (3) | 48–71 | F (n=3), M (n=1) | Back pain or incidental finding | 2–6 | N/A | Resection | NED, 18–96 | N/A | Thrombi, component of cavernous hemangioma |
| 5–6 | O'Neill, 2016 (2) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 7 | Bean, 2018 (4) | 66 | M | Incidental | 1.8 | Growing over 7 yrs | Resection | N/A | N/A | Thrombi, EMH, sclerosis; GNA14 mutation |
| 8 | Peng, 2017 (5) | 57 | F | Incidental | 3.3 | N/A | Resection | N/A | MR: peripheral enhancement with unenhanced center | – |
| 9 | Gonzalez, 2019 (6) | 80 | F | Gallbladder carcinoma | 2 | N/A | Resection | NED, 18 | CT: persistent hyperenhancement | Thrombi |
| 10 | Lunn, 2019 (7) | 67 | F | Low back pain | 2.9 | 5 mm in 1 yr | Biopsy | NED, 18 | MR: homogeneous persistent enhancement, hyperintensity on both DWI and ADC | – |
| 11 | Lunn, 2019 (7) | 33 | F | Mid-epigastric pain | 5.1 | N/A | Resection | N/A | CT, MR: peripheral enhancement with unenhanced center, hyperintensity on DWI, hypointensity on ADC | – |
| 12 | Lunn, 2019 (7) | 77 | M | Workup for recurrent infection | 2 | N/A | Biopsy | N/A | US: hypoechoic | – |
| CT, MR: rim-like enhancement with filling, peripheral hyperintensity on DWI | ||||||||||
| 13 | Lunn, 2019 (7) | 2 | M | Incidental | 1.9 | N/A | Resection | N/A | US: hypoechoic, heterogeneous | – |
| CT, MR: rim-like enhancement with filling, peripheral hyperintensity on DWI | ||||||||||
| 14 | Lunn, 2019 (7) | 48 | M | Lymphoma | 1.7 | N/A | Resection | N/A | US: peripherally hypoechoic | – |
| CT: peripheral enhancement | ||||||||||
| 15 | Merritt, 2019 (8) | 56 | M | Post-resection of GIST | 3.4 | N/A | Biopsy | N/A | US: peripherally hypoechoic, vascularity | – |
| MR: homogeneous persistent enhancement | ||||||||||
| 16–17 | Liau, 2020 (9) | 62, 46 | F | N/A | 1.2, 4.5 | N/A | N/A | N/A | N/A | Thrombi, EMH, sclerosis; GNA11 or GNA14 mutation |
| 18 | Rogers, 2022 (10) | 52 | F | Liver cirrhosis | 0.8 | 8 mm in 1.5 yrs | Biopsy + ablation | NED, 12 | MR: homogeneous enhancement (initial)→rim-like enhancement (follow-up), no restricted diffusion | – |
| 19 | Yang, 2023 (11) | 59 | F | N/A | 10 | N/A | Resection | NED, 12 | CT, MR: peripherally enhancement | – |
| 20 | Chua, 2022 (12) | 32 | F | End-stage renal disease | N/A | N/A | Biopsy | N/A | CT: hyperenhancement | – |
| 21 | Shanbhogue, 2022 (13) | 28 | F | N/A | 4 | N/A | N/A | N/A | US: hypoechoic, vascularity | – |
| MR: homogeneous persistent enhancement | ||||||||||
| 22 | Shanbhogue, 2022 (13) | 60 | M | N/A | N/A | Rapidly growing in 2 yrs | Biopsy + ablation | N/A | MR: homogeneous enhancement (initial)→peripheral enhancement with unenhanced center (follow-up) | – |
| 23 | Ma, 2023 (14) | 29 | M | Incidental | 2.1 | 32 mm in 3 yrs | Resection | N/A | US: peripherally hypoechoic (follow-up) | – |
| CT: hyperenhancement | ||||||||||
| MR: peripheral enhancement with complete filling, mild hyperintensity on DWI and ADC | ||||||||||
| 24 | Presented study | 50 | F | Incidental | 2.5 | 25 mm in 7 yrs | Resection | NED, 6 | US: mixed-echoic; dotted blood flow signals | Thrombi, EMH |
| MR: rim-like enhancement with partial filling, hyperintensity on DWI and ADC |
F, female; M, male; N/A, not available; NED, no evidence of disease; EMH, extramedullary hematopoiesis; yr, year; MR, magnetic resonance; CT, computed tomography; US, ultrasound; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient.
Of the reported cases of hepatic AH, approximately 80% are asymptomatic and incidentally detected on imaging workup for unrelated diseases. Symptoms are often nonspecific, such as back or epigastric pain (3,7). Age of initial detection ranges from 22 months to 80 years (the median age is approximately 56 years). Hepatic AHs seem to have a female predominance (female to male ratio: 1.8:1). Both right and left lobes can be involved. AHs tend to occur more frequently in a nonsubcapsular location (nonsubcapsular to subcapsular ratio: 1.7:1). Most cases are solitary, and 2 cases of multiple lesions have been reported (7,12). The diameters of reported hepatic AHs range from 0.8 to 10 cm.
On ultrasound, the tumor is usually observed as a well-defined mass with varied echogenicity (7,13,14). Unlike the more prevalent cavernous hemangioma, hypoechoic areas are often present within this entity. The color Doppler imaging features can range from small amounts of dotted blood flow signals to intense vascularity. On computed tomography (CT), the lesions show iso- or hypodensity and display persistent uniform enhancement or peripheral rim-like enhancement with centripetal filling (6,7,11,12,14). Calcifications and necrosis are rarely observed. On MRI, hepatic AHs typically demonstrate high intensity on T2WI and low intensity on T1WI, with similar enhancing presentation to that on CT (5,7,8,10,11,14). The most common enhancement patterns of reported hepatic AHs can be summarized into 2 distinct types: the first is homogeneous persistent hyperenhancement during arterial phase without subsequent washout in the portal venous or equilibrium phases (7,8,10,13), and the second type is a thick, continuous, rim-like enhancement in the arterial phase with progressively centripetal filling during the portal venous and equilibrium phases (5,7,11,14); the latter is consistent with our case. Central unenhanced areas in equilibrium or delayed phases are hypothesized to result from thrombosis or hyaline degeneration. However, no reported cases of hepatic AH have presented a nodular discontinuous peripheral enhancement during the arterial phase, which has been indicated to be the typical enhancement pattern of hepatic cavernous hemangioma. The lesions exhibit hypointensity in the hepatobiliary phase. There have been few published reports regarding the presentation of hepatic AHs on diffusion-weighted imaging (DWI) (7,10). Lunn et al. reported restricted diffusion in hepatic AHs (7). However, in the 4 cases of their study, only 1 case showed high signal on DWI with low signal on the ADC map; another patient exhibited slight hyperintensity on both DWI and ADC, which was consistent with the diffusion features of our cases and was likely due to the T2 shine-through effect rather than true restricted diffusion; ADC maps of the remaining 2 cases were not provided. In the case reported by Rogers et al., hepatic AH demonstrated no restricted diffusion (10), which is similar to a recently published case of retroperitoneal AH (15). In a previously published hepatic AH case at our hospital (14), the lesion showed no obviously restricted diffusion, with a mean an ADC value of 1.81×10-3mm2/s. Previous studies have reported that ADC values of hepatic hemangiomas [approximately (1.9–2.3)×10−3 mm2/s], including hemangiomas with both typical and atypical enhancement, were significantly larger than those of other hypervascular lesions, such as hepatocellular carcinoma, focal nodular hyperplasia, and neuroendocrine tumor (16,17). The ADC value of hepatic AH in our report is similar to those of previously documented hepatic hemangiomas. We speculate that despite the atypical enhancement pattern of hepatic AHs, a similar presentation to typical hepatic hemangiomas on DWI and ADC map may provide additional diagnostic indications of the benign vascular nature of this rare tumor.
AHs in the liver share the same histopathological features with the tumors in other sites. The tumor is typically made up of anastomosing small-caliber capillaries within a framework of supportive stroma. These vascular spaces are lined by a single layer of flat of cuboidal or oval endothelial cells which can exhibit nuclear hobnailing (1,2). Tumor cells are bland and show no or mild focal nuclear atypia, with mitoses being rare or absent. Fibrin thrombi, extramedullary hematopoiesis, and focal degeneration have been reported in a considerable subset of cases (3,4,6,9) and was encountered in our case. Tumor cells may, on rare occasions, contain hyaline globules, similar to those in Kaposi sarcoma (1). However, no case in the liver has been reported to involve this finding. On immunohistochemistry, AHs show diffusely positive expression of vascular endothelial markers (i.e., CD34, CD31, ERG, FLi-1, and Factor VIII) in the tumor cells. Some cases may also show smooth muscle actin (SMA)- or epithelial membrane antigen (EMA)-positive staining in supportive stroma. Genetic studies of AH have revealed recurrent activating mutations in GNAQ, GNA11, and GNA14 (G protein subunit alpha Q, 11 and 14) in over 90% of the investigated cases in the genitourinary system, soft tissue, and bones (4,9). These mutations have also been found in other benign vascular tumors, but have not been reported in angiosarcomas. However, only 3 cases of hepatic AH in published literature were reported to exhibit GNA11 or GNA14 mutations due to the limited number of cases (4,9). The chief challenge in pathological diagnosis of hepatic AH may be its susceptibility to being confused with other vascular neoplasms. The absence of multilayer endothelium, nuclear atypia, active mitosis, and invasive growth can help distinguish AH from vascular malignancies, such as angiosarcoma. Another benign vascular tumor, hepatic small vessel neoplasm (HSVN), exhibits similar morphological characteristics to those of AH. However, HSVNs have an infiltrative growth pattern without a well-defined margin against the surrounding parenchyma, which is unlike the typical findings in reported hepatic AHs (18).
The prognosis for this tumor type is generally good. Approximately two-thirds of cases of hepatic AH undergo surgical resection, and no recurrence or metastasis has been reported in postoperative follow-up ranging from 6 to 96 months (3,6,11). In two 2 cases of hepatic AH diagnosed with biopsy and treated with microwave ablation, there was no evidence of tumor recurrence during follow-up (10,13). There have been no reported hemorrhagic complications (neither spontaneous nor postbiopsy) (2). Information regarding the normal growth dynamics of hepatic AHs is sparse (7,10,13,14). The growth rates reported in 3 published cases with quantitative records were approximately 5–10 mm per year (7,10,14); another case displayed rapid tumor progression during a 2-year interval, but detailed size measurements were not provided (13). Our present case showed tumor enlargement during follow-up at a growth rate of 3.6 mm per year. AHs in other sites (soft tissue, kidney, intracranial) exhibited varied growth dynamics, from remaining stable to growing rapidly (19,20). A previous study indicated that almost 40% of conventional hepatic hemangiomas increased in size over time with an overall rate of 0.3–2 mm per year in diameter (21). Based on the limited available information, it appears that some hepatic AHs may grow more quickly than do conventional hepatic hemangiomas. The relatively rapid tumor growth may raise concerns about malignancy or complications and ultimately prompt surgeons to consider surgery. However, the lack of aggressive features on imaging, such as invasive growth, marked necrosis, prominent restricted diffusion, and metastasis, may indicate its benign nature.
Based on a review of prior literature and the current case, we believe that although certain hepatic AHs may show rapid lesion growth, several clinical and imaging features can signal radiologists and surgeons to the likelihood of this benign vascular tumor, including an incidental solitary mass with a well-defined margin, an “atypical” hemangioma enhancement pattern (i.e., homogeneous or continuous rim-like hyperenhancement without washout), and no or mild restricted diffusion on DWI and ADC map. In such cases, surveillance may be an appropriate option to avoid unnecessary surgery. In the future, it is necessary to collect more clinicopathological data and sequential radiological records of hepatic AH to gain a better understanding of this tumor’s natural course and related factors.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-485/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol 2009;33:1364-9. [Crossref] [PubMed]
- O'Neill AC, Craig JW, Silverman SG, Alencar RO. Anastomosing hemangiomas: locations of occurrence, imaging features, and diagnosis with percutaneous biopsy. Abdom Radiol (NY) 2016;41:1325-32. [Crossref] [PubMed]
- Lin J, Bigge J, Ulbright TM, Montgomery E. Anastomosing hemangioma of the liver and gastrointestinal tract: an unusual variant histologically mimicking angiosarcoma. Am J Surg Pathol 2013;37:1761-5. [Crossref] [PubMed]
- Bean GR, Joseph NM, Folpe AL, Horvai AE, Umetsu SE. Recurrent GNA14 mutations in anastomosing haemangiomas. Histopathology 2018;73:354-7. [Crossref] [PubMed]
- Peng X, Li J, Liang Z. Anastomosing haemangioma of liver: A case report. Mol Clin Oncol 2017;7:507-9. [Crossref] [PubMed]
- Gonzalez SP, Wachtel MS, Onkendi EO. Operative Management of T1b Gallbladder Carcinoma with Concurrent Hepatic Anastomosing Hemangioma. Cureus 2019;11:e5081. [Crossref] [PubMed]
- Lunn B, Yasir S, Lam-Himlin D, Menias CO, Torbenson MS, Venkatesh SK. Anastomosing hemangioma of the liver: a case series. Abdom Radiol (NY) 2019;44:2781-7. [Crossref] [PubMed]
- Merritt B, Behr S, Umetsu SE, Roberts J, Kolli KP. Anastomosing hemangioma of liver. J Radiol Case Rep 2019;13:32-9. [Crossref] [PubMed]
- Liau JY, Tsai JH, Lan J, Chen CC, Wang YH, Lee JC, Huang HY. GNA11 joins GNAQ and GNA14 as a recurrently mutated gene in anastomosing hemangioma. Virchows Arch 2020;476:475-81. [Crossref] [PubMed]
- Rogers T, Shah N, Mauro D, McGinty KA. Anastomosing hemangioma of the liver: An unusual variant in abdominal MRI imaging. Radiol Case Rep 2022;17:4889-92. [Crossref] [PubMed]
- Yang L, Han P, Liu X, Zhang Y. Easily confused with hepatic angiosarcoma: Rare hepatic giant anastomosing hemangioma. Asian J Surg 2023;46:1006-7. [Crossref] [PubMed]
- Chua WM, Hoe KMJ, Dalan R, Too CW, Ong SYK, Tay TKY, Loke KSH. Anastomosing Hemangioma on 68Ga-DOTATATE PET/CT: A Potential Pitfall. Clin Nucl Med 2022;47:321-3. [Crossref] [PubMed]
- Shanbhogue K, Khandelwal A, Hajdu C, Cao W, Surabhi VR, Prasad SR. Anastomosing hemangioma: a current update on clinical, pathological and imaging features. Abdom Radiol (NY) 2022;47:2335-46. [Crossref] [PubMed]
- Ma Y, Cao H, Wang H, Qiu L, Shang K, Wen Y. A growing liver anastomosing hemangioma. Quant Imaging Med Surg 2023;13:3365-70. [Crossref] [PubMed]
- Xue X, Song M, Xiao W, Chen F, Huang Q. Imaging findings of retroperitoneal anastomosing hemangioma: a case report and literature review. BMC Urol 2022;22:77. [Crossref] [PubMed]
- Nam SJ, Yu JS, Cho ES, Kim JH, Chung JJ. High-flow haemangiomas versus hypervascular hepatocellular carcinoma showing "pseudo-washout" on gadoxetic acid-enhanced hepatic MRI: value of diffusion-weighted imaging in the differential diagnosis of small lesions. Clin Radiol 2017;72:247-54. [Crossref] [PubMed]
- Vossen JA, Buijs M, Liapi E, Eng J, Bluemke DA, Kamel IR. Receiver operating characteristic analysis of diffusion-weighted magnetic resonance imaging in differentiating hepatic hemangioma from other hypervascular liver lesions. J Comput Assist Tomogr 2008;32:750-6. [Crossref] [PubMed]
- Goh IY, Mulholland P, Sokolova A, Liu C, Siriwardhane M. Hepatic small vessel neoplasm - A systematic review. Ann Med Surg (Lond) 2021;72:103004. [Crossref] [PubMed]
- John I, Folpe AL. Anastomosing Hemangiomas Arising in Unusual Locations: A Clinicopathologic Study of 17 Soft Tissue Cases Showing a Predilection for the Paraspinal Region. Am J Surg Pathol 2016;40:1084-9. [Crossref] [PubMed]
- Bodman A, Goodman A, Olson JJ. Intracranial thrombosed anastomosing hemangioma: Case report. Neuropathology 2020;40:206-10. [Crossref] [PubMed]
- Hasan HY, Hinshaw JL, Borman EJ, Gegios A, Leverson G, Winslow ER. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg 2014;149:1266-71. [Crossref] [PubMed]


