
The continuous treatment of anterior segment open globe injury: an eye injury vitrectomy study
Introduction
Open globe injury (OGI) is one of the major causes of irreversible visual impairment worldwide (1). It is defined according to the Birmingham Eye Trauma Terminology System (BETTS) and new proposed classification as a full-thickness wound of the eye wall with the mechanisms of injury including rupture, penetration, intraocular foreign body, perforation, and mixed injury (2,3). It can cause various types of damage to the intraocular structures and involve the anterior segment (AS), posterior segment (PS), or both. Open injuries of the AS accounted for about 80% of ocular injuries, which consisted of full-thickness lacerations of the corneal and the anterior sclera within 5 mm behind the limbus (4-6).
The human wound healing process is classically characterized into three stages: inflammation, proliferation, and remodeling (7,8). These stages culminate in scar formation (9). Primary repair of OGI consists of suturing the corneal and/or scleral wounds to provide a watertight closure and excising or reducing the prolapsed ocular contents. Most ophthalmic practitioners prefer initially performing a primary repair of the wounds to restore the integrity of the globe and normal intraocular pressure (IOP), followed by a delayed secondary procedure, if needed (10). The next step of the classic treatment for most cases is to wait for the natural healing process of the injured eye.
The prognostic significance of debridement has long been demonstrated for trauma other than intraocular tissues, for instance, for general surgery (11) of oral and maxillofacial, osteopathic (12,13), gynecological (14-16), and dermatologic tissue (17). For injuries involving PS, a vitrectomy is often applied to treat the eye injury. A vitrectomy in open traumatized eyes consists of intraocular debridement. Its main purpose is to remove vitreous hemorrhage and vitreous gel, reattach the detached retina, and clear the complex mass on the wound site because these factors are considered the basis for traumatic proliferative vitreoretinopathy (tPVR) (18-23). tPVR is an undesirable abnormal proliferation and a devastating complication in open injured eyes. After an OGI, the timing of a vitrectomy is urgent because the vitrectomy is closely associated with tPVR. It has been reported that vitrectomy performed more than 28 days after an OGI is more likely to lead to loss of the traumatized eye (24).
There are reasons to believe that the wound-healing process in the AS should follow that of PS. Some posttraumatic complications, such as adhesive corneal leukoma, secondary glaucoma, iris-pupil adherence, and ciliary epithelium detachment, generally occur because of malformed healing of disorganized tissues induced by AS injury, especially around the site of the wound. Unfortunately, the process of wound healing in the AS has not received as much attention as that of PS. According to previous studies of AS injury, the performance of pars plana vitrectomy, aphakia after initial trauma, loss of iris tissue and ciliary body damage, for instance, were associated with poor visual outcome (25,26). Continuous surgical treatment (CST), on the other hand, was not drawn attention to in any studies before. More research is needed to assess whether CST benefits the prognosis of patients with AS by debriding and reconstructing disorganized tissues before scar formation occurs. Continuous treatment of AS in cases of OGI can be used to interrupt this unfavorable healing process. CST is defined as anterior vitreous surgery within 4 weeks after the initial wound repair of the injury. To our knowledge, previous studies have not investigated this subject.
Our study aims to explore whether timely and proper continuous treatment can improve the prognosis of AS OGIs by comparing the ocular structure and visual function. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-645/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is a sub-study of the eye injury vitrectomy study (EIVS), which is a multi-center study led by Peking University Third Hospital. The study was approved by Peking University Third Hospital Ethics Committee (No. IRB00006761-2012060) and Peking University International Hospital was informed and agreed with the study. Informed consent was taken from all the patients.
Patients involved in this prospective comparative cohort study were recruited from January 1, 2020 to July 31, 2021 with an experience of AS OGI. The exclusion criteria included: (I) less than 6-month follow-up period; (II) previous ocular surgery; and (III) missing information on initial or final visual acuity (VA). Nine patients who had an OGI involved in the AS received an anterior vitrectomy (1 patient) or AS treatment as part of a pars plana vitrectomy (8 patients) within 8 weeks after initial wound repair. These 9 patients’ ocular conditions were consistent with the timing and indications for OGI vitrectomy. Besides, the scar formation had not occurred. Ten patients who did not undergo surgical intervention after the initial wound repair but had the same other features as patients in group 1 were assigned to group 2. All group 1 patients were visited at 1 week, 4 weeks, 12 weeks, and 6 months after CST and the baseline clinical factors and research data were collected and evaluated by the same doctor.
Patients’ demographic information and injury-related clinical characteristics are summarized in Table 1. According to the Ocular Trauma Classification Group guidelines (27), wound location was divided into three zones as follows: zone I, injury within the cornea, including the limbal area; zone II, an anterior injury within 5 mm of the sclera (not extending into the retina); and zone III, a scleral injury that extended more than 5 mm posteriorly from the limbus. The zone of injury is defined according to the location of the most posterior aspect of the injury. Only zone I and zone II injuries were involved in this research because they were AS OGI.
Table 1
| Features | Group 1 (n=9) | Group 2 (n=10) | P value |
|---|---|---|---|
| Gender | 0.510 | ||
| Male | 6 (66.7) | 8 (80.0) | |
| Female | 3 (33.3) | 2 (20.0) | |
| Age (years) | 18.89±13.14 [6–44] | 22.30±12.17 [5–36] | 0.564 |
| Subtypes of open injury | 0.281 | ||
| Laceration | 7 (77.8) | 9 (90.0) | |
| Penetration | 2 (22.2) | 1 (10.0) | |
| Intraocular foreign body | 2 (22.2) | 0 (0.0) | |
| Wound location | 0.701 | ||
| Zone I | 7 (77.8) | 7 (70.0) | |
| Zone II | 2 (22.2) | 3 (30.0) |
Data are shown as n (%) or mean ± standard deviation [range]. Group 1: patients with AS open globe injuries who received CST; group 2: patients without CST after the initial wound repair. AS, anterior segment; CST, continuous surgical treatment.
The area of corneal leucoma was drawn and calculated with ImageJ software (National Institutes of Health, Bethesda, MD, USA). The ratio of the corneal leucoma area compared to the total cornea was expressed as a decimal in the calculation. The presumably significant complications in this study were protocoled and summarized (Table 2). The presence or absence of complications was evaluated as 1 or 0, respectively.
Table 2
| BCVA (Snellen) | Group 1 | Group 2 | Total | P value |
|---|---|---|---|---|
| Initial | 0.366 | |||
| <20/200 | 9 (100.0) | 8 (80.0) | 17 (89.5) | |
| 20/200–20/66.67 | 0 (0.0) | 1 (10.0) | 1 (5.3) | |
| >20/66.67 | 0(0.0) | 1 (10.0) | 1 (5.3) | |
| Total | 9 | 10 | 19 | |
| Post-treatment | 0.011 | |||
| <20/200 | 1 (11.1) | 8 (80.0) | 9 (47.4) | |
| 20/200–20/66.67 | 3 (33.3) | 1 (10.0) | 4 (21.1) | |
| >20/66.67 | 5 (55.6) | 1 (10.0) | 6 (31.6) | |
| Total | 9 | 10 | 19 |
Data are shown as n (%) or number of cases. Group 1: patients with AS open globe injuries who received CST; group 2: patients without CST after the initial wound repair. VA, visual acuity; BCVA, best corrected visual acuity; CST, continuous surgical treatment; AS, anterior segment.
Corneal astigmatism was measured using a Pentacam (Oculus, Wetzlar, Germany) confined to the central area in a 4-mm diameter. Refractive error for regular astigmatism was analyzed using the results of optometry tests. Hypotony was defined as an IOP equal or less than 6 mmHg combined with signs of blood-aqueous barrier breakdown, such as the Tyndall phenomenon in the anterior chamber or vitreous cavity.
Details of primary and subsequent surgical management, the duration of follow-up, best corrected visual acuity (BCVA), and IOP at the final visit were reviewed. BCVA was recorded by reading the Snellen chart if the patients could read the chart. The VA of finger counting, hand motion, or light perception were converted digitally into 20/1600, 20/3,200, and 20/3,200, respectively. All the patients were followed up for more than 3 months postoperatively.
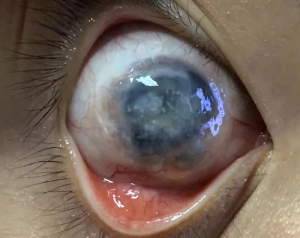
The pathologies in the AS, such as adhesive corneal leucoma, anterior/posterior synechia of iris, different depth of peripheral anterior chamber, severe destruction of AS, light pass through pupil, fibrosis/scar in the AS, and secondary glaucoma, were also reviewed (Table 3). Severe destruction of the AS was defined as the structures being highly disorganized and irreparable (Figure 1). Only 2 cases of group 2 had severe destruction of the AS. The light passing through the pupil was recorded as being blocked and unimpeded. In the last follow-up, fibrosis or scarring in the pupil area was used as a marker of residual fibrillation and/or scar tissue at the plane of the iris/lens.
Table 3
| Complications | Group 1 (n=9) | Group 2 (n=10) | P value |
|---|---|---|---|
| Adhesive corneal leucoma | 0 | 6 | 0.011 |
| Uneven anterior chamber by slit lamp/AS OCT/UBM | 1 | 6 | 0.022 |
| Blocking the light passing through the pupil | 0 | 3 | 0.037 |
| Fibrosis or scarring in the AS | 3 | 8 | 0.040 |
| Secondary glaucoma | 0 | 3 | 0.037 |
| Severe destruction of the AS | 0 | 2 | 0.474 |
Data are shown as number of cases. Group 1: patients with AS open globe injuries who received CST; group 2: patients without CST after the initial wound repair. AS, anterior segment; OCT, optical coherence tomography; UBM, ultrasound biomicroscope; CST, continuous surgical treatment.
The score of the AS injury was recorded as 100 points if there was no AS involved previously mentioned and listed in Table 3. With each involvement, we deducted 10 points, then calculated the score for each patient and compared the differences between the two groups.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (IBM, Armonk, NY, USA). The Student’s t-test was used to analyze continuous variables, such as differences in the VA outcome, corneal leucoma area ratio/astigmatism, and score of anterior injury between the continuous treatment group (treatment) and the initial treatment group (control). The chi-squared test or Fisher’s exact test were performed to compare demographic characteristics, injury-related features, and the occurrence of significant complications associated with AS injury between the two groups. P values less than 0.05 were considered statistically significant.
Results
The patients in group 1 (treatment) and group 2 (control) were comparable statistically in the aspects of gender, age, types of injury, and involved wound location (Table 1). The profiles of the VA outcomes by the end of the study are shown in Table 2. The initial VA of two groups was not statistically significant (P=0.366). The general trends in the final outcome in BCVA after intervention were statistically better in group 1 than in group 2 (P=0.011). The abnormalities and severe complications caused by AS injury are listed in Table 3. Adhesive corneal leucoma, an uneven anterior chamber, blocked light passing through the pupil, and fibrosis or scarring in the AS more frequently occurred in group 2 (P=0.011, 0.022, 0.037, and 0.040, respectively). Three cases of secondary glaucoma occurred in group 2 with a significant difference compared to group 1 (P=0.037). Two cases of severe AS structure destruction appeared in group 2, but the result was not statistically significant (P=0.474), possibly because of the limited sample size of this study. The area ratio of leucoma (0.79±0.44, 0.82±0.50, respectively) and corneal astigmatism (3.69±1.90, 4.50±4.80, respectively) revealed not statistically significant between the two groups (Table 4). On the other hand, the score of AS abnormalities, mean values being 93.33±11.18 for group 1 and 67.00±29.46 for group 2, was statistically different (P=0.022). As exhibited in Figure 2, group 1 benefitted more from CST than group 2.
Table 4
| Variables | Group 1 | Group 2 | P value |
|---|---|---|---|
| Area ratio (ratio of leucoma and the whole cornea) | 0.79±0.44 | 0.82±0.50 | 0.897 |
| Corneal astigmatism | 3.69±1.90 | 4.50±4.80 | 0.671 |
| Score of AS abnormalities | 93.33±11.18 | 67.00±29.46 | 0.022 |
Data are shown as mean ± standard deviation. Group 1: patients with AS open globe injuries who received CST; group 2: patients without CST after the initial wound repair. AS, anterior segment; CST, continuous surgical treatment.
Discussion
The benefits of debridement are well-established in other surgical fields, with the exception of intraocular settings (11-17). Ophthalmologists have been conducting debridement in open injured eyes even though there are no formulated guidelines for the procedure. However, the wound-healing processes of ocular tissues show the similarity of the characteristics to other tissues (8). The main principle of general surgery is to remove contaminated or infectious necrotic tissue (28). However, intraocular debridement differs from other tissues after injury because the intraocular tissue is not infected. Instead, intraocular debridement aims to eliminate the causes of persistent inflammation and abnormal proliferation.
With recent advances in molecular, genetic, and imaging techniques, the endogenous electric fields are considered as key role in wound healing processes. Electric fields are along consistently with the repair process and are considered overriding guidance role in directing cell migration in epithelia wound healing. The current emittance after the tail of the tadpole is cut off lasts 8–10 hours. If this residue is renewable, it will generate an inward current flow that regenerates throughout the process (29). Thus, if the repair process of injured tissue is not completed, the electric fields governing cell migration and proliferation will not terminate (30). These findings suggest that curtailing the chronic repair process of damaged tissue as early as possible can reduce long-term complications caused by abnormal proliferation.
In term of histology of wound repair and regeneration, the concept of cellular patterning suggests that the repair results of injured tissue depend on the spatiotemporal conditions of the environment in which they live (8,29). The purpose of CST for AS OGI is not to remove the necrotic tissue/infectious tissue as does in general surgeries, but to improve the prognosis of the injured eye by eliminating structurally disorganized tissues. This can be done by dissecting the connection with the inner site of the wound tract, restoring the residual AS tissue, and improving the local repair environment.
The 3 stages of wound healing of ocular tissues are inextricably linked to the extracellular matrix in each stage (7). The breakdown of extracellular matrix is essential for wound healing and scar tissue formation. Tissue repair commences immediately following injury and most often culminates in the replacement of damaged tissue with an acellular fibrotic matrix (i.e., scar tissue). In other words, abnormal healing of the wound after injury may cause even worse consequences due to scar tissue formation. Possible consequences include adhesive corneal leucoma and synechia of the iris, insufficient transparency of the refracting media, and the uneven depth of the peripheral anterior chamber that could damage the aqueous humor drainage system. Prolonged inflammation and insufficient resurfacing exacerbate excessive collagen deposition, thus leading to impaired wound healing (i.e., fibrosis), which impedes functional recovery. These consequences can be prevented with CST for the open injury in the AS at the proper time after primary wound repair and before scar formation.
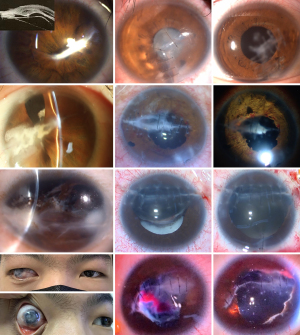
Figures 3-5 show various stages in the three injured eyes at time intervals of 13 days, 45 days, and 6 months between the injury and surgery. These figures show the injured tissues eventually lead to fibrosis or scarring in the AS, the severity of the fibrosis and scarring in disorganized tissues and the wound site deteriorated over time after injury. The abnormal proliferation and wound healing processes were synchronized in the AS and PS.

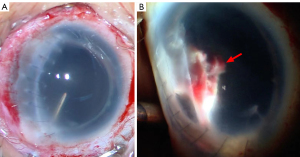

A review of 1,375 enucleations in the TongRen eye center in Beijing, China, showed that ocular trauma was the leading cause of these enucleations (31). The corneal-scleral wounds accounted for 40.4% of the enucleations. Abnormal histopathological findings in the AS showed anterior synechiae and secondary angle closure (46.9%), complicated with glaucomatous cupping of the optic nerve head (40.1%), pupillary membranes (14.3%), and anterior chamber membranes (13.5%), and intraocular inflammatory reactions (10.9%). These results demonstrated that AS involvement in OGI accounted for many injuries, and the prognosis could be improved with a better treatment regime.
A review by Cleary et al. (22) and Winthrop et al. (32) in 1979–1980 showed that many trauma-caused AS abnormalities had a posterior penetrating injury. These injuries included the ciliary processes being drawn up to the wound, the fibrous tissue forming a cyclitic membrane, and the proliferation of the non-pigmented ciliary epithelium 21 days after the injury (22). The condensed vitreous fibrils became incorporated into the peripheral corneal wound (32). These dynamic observations in the experiment were compared with the enucleated eye globes that resulted from injury (31). We considered that CST of the AS in conjunction with a vitrectomy improved the prognosis according to these reports.
Similar to the treatment of open injuries in the PS, the timing of continuous treatment is imperative to prevent complications in the AS after injury. Historically, the timing of a vitrectomy for open injuries in the PS has been controversial (33-37). Based on the EIVS results from a prospective cohort multicenter clinical study, vitrectomy should be carried out no later than 4 weeks after the injury is sustained (24). Similar results were concluded in a clinical histopathological investigation (38). In Figure 3, the optimal timing (13 days) for CST was implemented after the injury. Figure 5 shows a case in which the operation on the injured eye occurred after the optimal timing. Any change to a clinical routine must be based on evidence.
We compared the incidence of disorganized structure in the AS between the two groups. Optimally-timed CST reduced the chronic inflammation and rebuilt the wound surface. This resulted in less adhesive corneal leucoma and synechia of the iris. In addition, the amount of light passing through the pupil and the depth of the peripheral anterior chamber were less affected.
We initially presumed that the area of corneal scar should be larger in group 2, since more adherent corneal leucoma showed up in this group, which can induce more extensive opacity. The statistical analysis did not show a significant difference between the two groups. Adherent corneal leucoma occurred in 6 cases in group 2, while there were no manifestations in group 1 (chi-squared test, P<0.05). The difference between two analytic results could be attributed to the accuracy of the manually-drawn measurements for the opacity area. More precise results can be obtained using anterior optical coherence tomography (OCT) and expanding the sample size. Three cases of secondary glaucoma occurred in group 2, while patients in group 1 had normal IOP 1 (P<0.05). One case with 6–9 mmHg ocular hypotony also occurred in group 2. Our study demonstrated that CST prevented long-term complications.
Eight cases in group 1 received continuous treatment of the AS combined with vitreous surgery. The time intervals between initial wound repair and pars plana vitrectomy were less than 14 days in 3 cases, while the intervals were more than 4 weeks but less than 8 weeks in 5 cases. There were no differences among the 8 cases in terms of their recovery profile based on an AS assessment. Our study demonstrated that CST of the AS in conjunction with a vitrectomy improved the prognosis.
Our study had limitations. This study only included two centers and contained a relatively small sample size. If the sample size is expanded, some different results could be revealed, such as ratio of keratoleucoma area/total of cornea.
Conclusions
The benefits and necessity of wound debridement have been established in general surgery and other surgical subspecialties. However, techniques for the debridement of AS OGI have not been standardized. The nature course of wound healing in AS follows general rule in intraocular tissues and the pathological processes are synchronized with the wound healing in PS. Our study shows that CST initiated before scar formation can prevent complications when treating open AS injuries. The results of our study supply evidence for a standardized clinical routine to manage open AS injuries. Multicenter trials and sample expansion are needed for further investigations.
Acknowledgments
Funding: The study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-645/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-645/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Peking University Third Hospital Ethics Committee (No. IRB00006761-2012060) and Peking University International Hospital was informed and agreed with this study. Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Madhusudhan AP, Evelyn-Tai LM, Zamri N, Adil H, Wan-Hazabbah WH. Open globe injury in Hospital Universiti Sains Malaysia - A 10-year review. Int J Ophthalmol 2014;7:486-90. [Crossref] [PubMed]
- Scott R. The Ocular Trauma Score. Community Eye Health 2015;28:44-5.
- Feng K, Yao Y, Wang ZJ, Nie HP, Pang XQ, Chen HJ, Jiang YR, Hu YT, Ma ZZ. Mechanism and prognostic indicators for explosion-related eye trauma: eye injury vitrectomy study. Acta Ophthalmol 2021;99:e956-62. [Crossref] [PubMed]
- D'Antone VA, Cely Quiroz L, Palencia Florez DC. Clinical profile of ocular injuries in a geographically isolated Colombian municipality. Int Emerg Nurs 2020;52:100909. [Crossref] [PubMed]
- Barry RJ, Sii F, Bruynseels A, Abbott J, Blanch RJ, MacEwen CJ, Shah P. The UK Paediatric Ocular Trauma Study 3. (POTS3): clinical features and initial management of injuries. Clin Ophthalmol 2019;13:1165-72. [Crossref] [PubMed]
- Omar R, Anan NS, Azri IA, Majumder C, Knight VF. Characteristics of eye injuries, medical cost and return-to-work status among industrial workers: a retrospective study. BMJ Open 2022;12:e048965. [Crossref] [PubMed]
- Keane TJ, Horejs CM, Stevens MM. Scarring vs. functional healing: Matrix-based strategies to regulate tissue repair. Adv Drug Deliv Rev 2018;129:407-19. [Crossref] [PubMed]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314-21. [Crossref] [PubMed]
- Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle) 2015;4:119-36. [Crossref] [PubMed]
- American Association of Ophthalmology. Basic and Clinical Science Course. Section 12 Retina and Vitreous. 2021-2022;2021:358-59.
- Dryburgh N, Smith F, Donaldson J, Mitchell M. Debridement for surgical wounds. Cochrane Database Syst Rev 2008;CD006214. [Crossref] [PubMed]
- Liodakis E, Bruns N, Macke C, Krettek C, Omar M. 3D-printed template-assisted reduction of long bone fractures. Unfallchirurg 2019;122:286-92. [Crossref] [PubMed]
- Doll J, Waizenegger S, Schmidmaier G, Weber MA, Fischer C. Contrast-Enhanced Ultrasound: A Viable Diagnostic Tool in Predicting Treatment Failure after Non-union Revision Surgery for Upper- and Lower-Limb Non-unions. Ultrasound Med Biol 2021;47:3147-58. [Crossref] [PubMed]
- Karmisholt KE, Taudorf EH, Wulff CB, Wenande E, Philipsen PA, Haedersdal M. Fractional CO(2) laser treatment of caesarean section scars-A randomized controlled split-scar trial with long term follow-up assessment. Lasers Surg Med 2017;49:189-97. [Crossref] [PubMed]
- Osterhoff G, Noser J, Held U, Werner CML, Pape HC, Dietrich M. Early Operative Versus Nonoperative Treatment of Fragility Fractures of the Pelvis: A Propensity-Matched Multicenter Study. J Orthop Trauma 2019;33:e410-5. [Crossref] [PubMed]
- Zhang NN, Wang GW, Zuo N, Yang Q. Novel laparoscopic surgery for the repair of cesarean scar defect without processing scar resection. BMC Pregnancy Childbirth 2021;21:815. [Crossref] [PubMed]
- Cen S, Huang F, Yang T, Tu C, Xiang Z, Liu L, Fang Y, Wang G, Zhang H, Zhou Z, Yi M, Duan H, Pei F. Early use of vacuum sealing drainage to repair the wound of the injured in Wenchuan earthquake. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009;23:657-9.
- Coles WH, Haik GM. Vitrectomy in intraocular trauma. Its rationale and its indications and limitations. Arch Ophthalmol 1972;87:621-8. [Crossref] [PubMed]
- Topping TM, Abrams GW, Machemer R. Experimental double-perforating injury of the posterior segment in rabbit eyes: the natural history of intraocular proliferation. Arch Ophthalmol 1979;97:735-42. [Crossref] [PubMed]
- Cleary PE, Ryan SJ. Vitrectomy in penetrating eye injury. Results of a controlled trial of vitrectomy in an experimental posterior penetrating eye injury in the rhesus monkey. Arch Ophthalmol 1981;99:287-92. [Crossref] [PubMed]
- Cleary PE, Ryan SJ. Experimental posterior penetrating eye injury in the rabbit. I. Method of production and natural history. Br J Ophthalmol 1979;63:306-11. [Crossref] [PubMed]
- Cleary PE, Ryan SJ. Experimental posterior penetrating eye injury in the rabbit. II. Histology of wound, vitreous, and retina. Br J Ophthalmol 1979;63:312-21. [Crossref] [PubMed]
- Tolentino FI, Liu HS, Freeman HM, Natchiar G. Vitrectomy in penetrating ocular trauma: an experimental study using rabbits. Ann Ophthalmol 1979;11:1763-71.
- Feng K, Hu Y, Wang C, Shen L, Pang X, Jiang Y, Nie H, Wang Z, Ma Z. Risk factors, anatomical, and visual outcomes of injured eyes with proliferative vitreoretinopathy: eye injury vitrectomy study. Retina 2013;33:1512-8. [Crossref] [PubMed]
- Feng K, Shen L, Pang X, Jiang Y, Nie H, Wang Z, Hu Y, Ma Z. Case-control study of risk factors for no light perception after open-globe injury: eye injury vitrectomy study. Retina 2011;31:1988-96. [Crossref] [PubMed]
- Gursoy H, Bilgec MD, Sahin A, Colak E. A Possible Regression Equation for Predicting Visual Outcomes after Surgical Repair of Open Globe Injuries. J Ophthalmol 2017;2017:1320457. [Crossref] [PubMed]
- Pieramici DJ, Sternberg P Jr, Aaberg TM Sr, Bridges WZ Jr, Capone A Jr, Cardillo JA, de Juan E Jr, Kuhn F, Meredith TA, Mieler WF, Olsen TW, Rubsamen P, Stout T. A system for classifying mechanical injuries of the eye (globe). The Ocular Trauma Classification Group. Am J Ophthalmol 1997;123:820-31. [Crossref] [PubMed]
- Anghel EL, DeFazio MV, Barker JC, Janis JE, Attinger CE. Current Concepts in Debridement: Science and Strategies. Plast Reconstr Surg 2016;138:82S-93S. [Crossref] [PubMed]
- Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol 2009;20:674-82. [Crossref] [PubMed]
- Wu DY, Pollock JD, Shurtleff D. Seminars in Cell & Developmental Biology. The molecular and cellular mechanisms in the development of addiction. Semin Cell Dev Biol 2009;20:377. Editorial. [Crossref] [PubMed]
- Cheng GY, Li B, Li LQ, Gao F, Ren RJ, Xu XL, Jonas JB. Review of 1375 enucleations in the TongRen Eye Centre, Beijing. Eye (Lond) 2008;22:1404-9. [Crossref] [PubMed]
- Winthrop SR, Cleary PE, Minckler DS, Ryan SJ. Penetrating eye injuries: a histopathological review. Br J Ophthalmol 1980;64:809-17. [Crossref] [PubMed]
- He Y, Zhang L, Wang F, Zhu M, Wang Y, Liu Y. Timing influence on outcomes of vitrectomy for open-globe injury: a prospective randomized comparative study. Retina 2020;40:725-34. [Crossref] [PubMed]
- Mieler WF, Mittra RA. The role and timing of pars plana vitrectomy in penetrating ocular trauma. Arch Ophthalmol 1997;115:1191-2. [Crossref] [PubMed]
- Thompson JT, Parver LM, Enger CL, Mieler WF, Liggett PE. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. National Eye Trauma System. Ophthalmology 1993;100:1468-74. [Crossref] [PubMed]
- Ghoraba HH, Heikal MA, Mansour HO, Abdelfattah HM, Elgemai EM, Zaky AG. Timing of Pars Plana Vitrectomy in Management of Gunshot Perforating Eye Injury: Observational Study. J Ophthalmol 2016;2016:1487407. [Crossref] [PubMed]
- Stepp MA, Menko AS. Immune responses to injury and their links to eye disease. Transl Res 2021;236:52-71. [Crossref] [PubMed]
- Jin Y, Chen H, Xu X, Hu Y, Wang C, Ma Z. Traumatic proliferative vitreoretinopathy: clinical and histopathological observations. Retina 2017;37:1236-45. [Crossref] [PubMed]


