
Both decreased and increased grey-to-white matter attenuation ratio in the putamen and caudate on early head computed tomography differentiate patients with favorable and unfavorable outcomes after prolonged cardiac arrest—secondary analysis of the Prague OHCA study
Introduction
Refractory out-of-hospital cardiac arrest (OHCA) is burdened with high mortality and poor neurological outcome (1). Two recent randomized controlled trials, ARREST and Prague OHCA study suggested reasonable neurologically favorable survival using an invasive approach including extracorporeal cardiopulmonary resuscitation (ECPR) despite prolonged periods of CPR (2,3). Still, neurological damage remains the leading cause of death in cardiac arrest victims with early neuroprognostication being the cornerstone of the decision-making process to continue or discontinue advanced treatments (4,5).
Brain computed tomography (CT) is an integral part of neuroprognostication after cardiac arrest, however, is not routinely recommended in all patients after OHCA. European Resuscitation Council and European Society of Intensive Care Medicine guidelines suggest brain imaging for an unknown cause of cardiac arrest and prognostication in centers with specific experience (6). Acute findings in OHCA patients are present in 40% and brain imaging provides information leading to changes in patients’ management in 16% within the first 2 days (7). The morphological hallmark of adverse neurological outcome is loss of cortical grey-white matter differentiation due to global cerebral edema, decreased attenuation of deep grey matter, and less frequently hemorrhage (7). Loss of grey-white matter differentiation on early brain CT is a reliable predictor of poor neurological outcome, in addition to predictors based on pre-hospital circumstances and in-hospital parameters (8,9). Brain CT imaging may be affected by previous contrast agent administration in patients managed with invasive assessment and therapy (i.e., coronary angiography/percutaneous coronary intervention) influencing grey matter attenuation in hypoxic-ischemic injury (10). All these predictors have already been evaluated in OHCA patients with sustained return of spontaneous circulation (7-10). Whether the same criteria may also be applied to refractory OHCA patients treated with ECPR has not been extensively studied yet (11-13).
Therefore, we analyzed a randomized population derived from the Prague OHCA trial to identify the signs of an adverse neurological outcome within 180 days in patients with refractory OHCA using brain CT in the first 36 hours after cardiac arrest.
Methods
Population and study design
This study is a secondary analysis of the Prague OHCA study, a randomized clinical trial conducted at a single center in Prague, Czech Republic, from March 1, 2013, to October 25, 2020. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The original study as well as secondary analyses were approved by the Ethics Committee of the General University Hospital in Prague (No. 192/11 S-IV) and informed consent was taken from all individual participants or their relatives.
Adult patients resuscitated for witnessed OHCA of presumed cardiac etiology after at least 5 minutes of advanced cardiovascular life support (ACLS) were eligible for enrollment in the trial. A web-based secured randomization system was used to assign patient number and intervention group during ongoing CPR in the field. The methodology and results of the intention to treat analysis were published in detail elsewhere (2,14). The Prague OHCA study database provided clinical and laboratory data (2).
In the current analysis, out of 256 patients included in the Prague OHCA study, we identified patients, who underwent brain CT within 36 hours after cardiac arrest (15,16). As a non-contrast brain CT was not part of the protocol, reasons for performing the examination have also been identified.
Brain CT acquisition and evaluation
Non-contrast brain CT was performed in a spiral mode on either a 256-slice (Brilliance iCT, Philips, Best, Netherlands) or 64-slice scanner (Somatom Definition, Siemens, Forchheim, Germany) with a peak voltage of 120 kV, tube time-current product between 189–349 mAs from the vertex to C1 vertebra. The images were reconstructed in 5 mm contiguous axial sections and analyzed. Briefly, CT images were assessed for the presence of intracranial hemorrhage, edema, and traces of contrast material following eventual previous contrast agent administration [attenuation in venous sinuses ≥70 Hounsfield unit (HU)] by two radiologists with clinical experience of 4 and 13 years in consensus, who were blinded to the clinical outcome. The quantitative analysis of the images involved measurement of attenuation of deep grey matter (caudate, putamen, thalamus), medial frontal and occipital cortex, and semioval center as a reference attenuation of the white matter (17). Attenuation was measured on a clinical workstation (Intellispace Portal, Philips, Best, The Netherlands) on 5 mm axial sections in a manually drawn spline area of interest (≥30 mm2, Figure S1). The measurements were repeated in ten randomly selected patients to quantify interobserver agreement. The measurements from bilateral structures were averaged and grey-to-white matter attenuation ratio (GWR) was calculated.
Outcomes
The primary outcome of the current analysis was the best neurological outcome achieved within 180 days after cardiac arrest. A cerebral performance category (CPC) of 1–2 was considered a good neurological outcome and a CPC of 3–5 was a poor neurological outcome (18).
Statistical analysis
Statistical analysis was performed in Prism (GraphPad Software, La Jolla, CA, USA) and R (R foundation for statistical computing, Vienna, Austria). The normality of the continuous data was tested using the D’Agostino & Pearson omnibus normality test. To test for statistical significance, we used the t-test, Mann-Whitney test, Fischer test, or χ2 test as appropriate. Two-sided attenuation thresholds were proposed to separate patients with CPC 1–2 and CPC 3–5 after inspection of the distribution of the data in a plot. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated. Receiver operator characteristic (ROC) curves were created and areas under the ROC curve (AUCs) were calculated using absolute difference from the average of the two proposed thresholds. Association between attenuation or GWR in grey matter structures and mean attenuation in the superior and inferior sagittal sinuses was calculated using Spearman’s rank correlation coefficient (rho). Interobserver agreement was expressed as an intraclass correlation coefficient (ICC). A P value <0.05 was considered significant.
Results
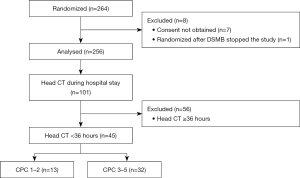
The Prague OHCA study database provided clinical data including the CPC shown in Table 1. Out of 256 patients enrolled in the main study, 101 (39%) had a CT of the brain performed during their hospital stay, and 45 of them within the first 36 hours after initial collapse, representing the current study population. There were 32 (71%) patients with unfavorable neurological outcomes (CPC 3 in a single patient, CPC 4 in 9 patients, CPC 5 in 22 patients) and 13 (29%) with favorable neurological outcomes (CPC 1 in 12 patients; CPC 2 in a single patient), see Figure 1. In patients with CPC 3–5, following causes of death were recorded: brain death in 13 (41%) patients, multiorgan failure in 12 (38%), cardiac rearrest in 4 (13%), hemorrhage in 2 (6%), and unknown cause in 1 (3%).
Table 1
| Characteristics | CPC 3–5 (n=32) | CPC 1–2 (n=13) | P |
|---|---|---|---|
| Gender (male) | 24 [75] | 8 [62] | 0.47 |
| Age (years) | 59.0±1.9 | 55.3±3.6 | 0.33 |
| Arterial hypertension | 17 [65] | 6 [46] | 0.31 |
| Diabetes | 7 [28] | 0 | 0.07 |
| Coronary artery disease | 5 [20] | 1 [8] | 0.64 |
| Shockable rhythm | 11 [34] | 13 [100] | <0.001* |
| pH on admission | 6.959±0.027 | 7.107±0.041 | 0.005* |
| Lactate on admission (mmol/L) | 12.72±0.76 | 8.25±0.95 | 0.002* |
| Duration of cardiac arrest (min) | 49±3 | 36±5 | 0.021* |
| ECPR | 24 [75] | 7 [54] | 0.29 |
| CPC (best CPC within 180 days) | – | ||
| CPC 1 | 12 [92] | ||
| CPC 2 | 1 [8] | ||
| CPC 3 | 1 [3] | ||
| CPC 4 | 9 [28] | ||
| CPC 5 | 22 [69] | ||
| Coronary angiography before head CT | |||
| Number of patients | 31 [97] | 11 [85] | 0.20 |
| Time before CT (hours) | 3.4 (1.4 to 19.5) | 2.8 (1.0 to 9.8) | 0.51 |
Values are expressed as number [%], mean ± standard deviation, or median (IQR). *, significant P values. CPC, cerebral performance category; ECPR, extracorporeal cardiopulmonary resuscitation; CT, computed tomography; IQR, interquartile range.
ECPR was performed in 24 (75%) patients with CPC 3–5 and 7 (54%) patients with CPC 1–2 (P=0.29). Patients with CPC 3–5 had lower pH (P=0.005), higher lactate (P=0.002), and longer duration of cardiac arrest (P=0.021). All but three patients (93%) had undergone coronary angiography before head CT.
CT examination was requested for adverse neurological status (n=22), unknown cause of cardiac arrest (n=15), craniocerebral trauma (n=5), or performed alongside CT of the thorax (n=3), see Table 2 for details. CT examinations were performed 4.3 [interquartile range (IQR), 2.5–14.4] hours after an emergency call.
Table 2
| CT | CPC 3–5 (n=32) | CPC 1–2 (n=13) | P |
|---|---|---|---|
| CT requested for | 0.012 | ||
| Adverse neurological status | 19 [59] | 3 [23] | |
| Unknown cause of cardiac arrest | 9 [28] | 6 [46] | |
| Craniocerebral trauma | 1 [3] | 4 [31] | |
| At the occasion of CT of the thorax or abdomen | 3 [9] | 0 [0] | |
| The time between cardiac arrest and CT (hours) | 4.8 (2.6 to 20.0) | 2.8 (2.2 to 10.4) | 0.37 |
| CT findings | |||
| Intracranial hemorrhage | 6 [19] | 0 [0] | 0.16 |
| Edema or conus | 16 [50] | 0 [0] | 0.0014* |
| Normal appearance | 14 [44] | 13 [100] | 0.0004* |
| Attenuation in sinus rectus ≥70 HU | 16 [50] | 6 [46] | 1.0 |
Values are expressed as number [%] or median (IQR). *, significant P values. CT, computed tomography; CPC, cerebral performance category; HU, Hounsfield unit; IQR, interquartile range.
High attenuation of dural venous sinuses attributable to residual contrast material after a previous angiography was present in 22 (49%) patients. A normal finding was reported in 14 (44%) patients with CPC 3–5 and all 13 patients with CPC 1–2 (P=0.0004). Intracranial edema (n=16) and hemorrhage (n=6) were present in patients with CPC 3–5 only.
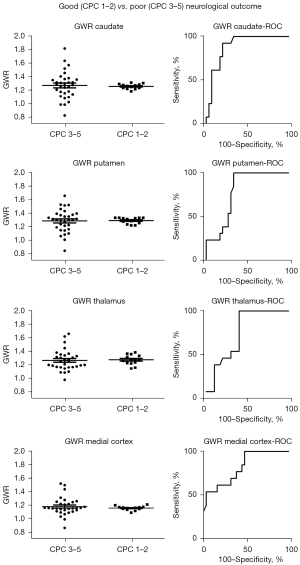
Attenuation of brain structures and GWR were not different between patients with favorable and unfavorable outcomes (Table S1, Figure S2). However, the GWR in the caudate and putamen of most CPC 1–2 patients was within a narrow range of values (1.18 to 1.30, n=11, 85% and 1.20 to 1.33, n=13, 100%, respectively) that separated CPC 1–2 and CPC 3–5 with a sensitivity of 78% and 66% a specificity of 85% and 100%, and AUC of 0.86 (P=0.0001) and 0.77 (P=0.0053), respectively (Table 3, Figures 2,3). These thresholds, however, did not differentiate patients treated by conventional vs. extracorporeal CPR (AUC =0.64, P=0.13 and AUC =0.66, P=0.082, respectively) (Table S1). Patients treated with ECPR had lower attenuation in the centrum semiovale (28.3±2.7 HU) compared to those without (31.0±2.8 HU, P=0.003) and marginally higher GWR in the thalamus [1.30 (IQR, 0.15) vs. 1.22 (IQR, 0.11), P=0.024] and medial cortex [1.16 (IQR, 0.09) vs. 1.13, (IQR, 0.10), P=0.021]. There was a positive association between mean attenuation in the superior and inferior sagittal sinus and attenuation in the putamen and the thalamus (Table S2). No significant correlation was found between time from the event and attenuation or attenuation ratios in the parenchymal brain structures, although attenuation of the venous sinuses was negatively correlated (−0.51, P=0.0003). The reliability of attenuation measurements was excellent (ICC was between 0.905 and 0.982).
Table 3
| Brain structure | Patients with CPC 3–5 (n=32) | Patients with CPC 1–2 (n=13) | P (CPC 3–5 vs. CPC 1–2) |
Selected threshold | Sensitivity (%) | Specificity (%) | PPV (%) |
NPV (%) |
AUC | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||||
| Panel A: attenuation (HU) | |||||||||||
| Caudate | 36.83±5.67 | 36.50±3.08 | 0.85 | 33 | 40 | 41 | 92 | 93 | 39 | 0.67 | 0.073 |
| Putamen | 37.05±4.60 | 37.42±2.81 | 0.79 | 33 | 39 | 41 | 69 | 76 | 32 | 0.57 | 0.48 |
| Thalamus | 35.0 (4.1) | 36.5 (3.0) | 0.15 | 33 | 40 | 22 | 85 | 78 | 31 | 0.58 | 0.42 |
| Medial cortex | 33.3 (4.9) | 32.5 (4.8) | 0.88 | 30 | 39 | 25 | 100 | 100 | 35 | 0.54 | 0.66 |
| CS | 28.8 (3.9) | 29.0 (4.0) | 0.99 | 27 | 33 | 28 | 62 | 64 | 26 | 0.51 | 0.95 |
| Panel B: attenuation ratio (GWR) | |||||||||||
| Caudate: CS | 1.27±0.20 | 1.25±0.04 | 0.81 | 1.18 | 1.30 | 78 | 85 | 93 | 61 | 0.86 | 0.0001* |
| Putamen: CS | 1.28±0.17 | 1.29±0.05 | 0.92 | 1.20 | 1.33 | 66 | 100 | 100 | 54 | 0.77 | 0.0053* |
| Thalamus: CS | 1.25 (0.16) | 1.28 (0.11) | 0.22 | 1.21 | 1.40 | 59 | 100 | 100 | 50 | 0.73 | 0.015* |
| Medial cortex: CS | 1.15 (0.12) | 1.16 (0.04) | 0.94 | 1.10 | 1.20 | 47 | 100 | 100 | 43 | 0.82 | 0.0008* |
Data are expressed as mean ± standard deviation or median (IQR). In this table, IQRs are expressed Q75 minus Q25. *, significant P values. GWR, grey-to-white matter attenuation ratio; CPC, cerebral performance category; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the ROC curve; ROC, receiver operator characteristic; HU, Hounsfield unit; CS, centrum semiovale; IQR, interquartile range; Q25, 25th percentile; Q75, 75th percentile.
Discussion
In this secondary analysis of the randomized Prague OHCA study, where patients were treated by both conventional and ECPR, we analyzed a potential prognostic value of non-contrast brain CT performed within 36 hours after prolonged cardiac arrest. We have identified lower and upper thresholds of GWR as a quantitative marker of hypoxic-ischemic brain injury that differentiated patients with favorable and unfavorable neurological outcomes. These thresholds did not distinguish patients with and without ECPR. Still, patients treated with ECPR demonstrated lower white matter attenuation. Nearly half of brain CTs showed residual contrast material contamination from previous coronary angiography. The attenuation in the venous sinuses correlated with attenuation in the putamen and the thalamus.
Despite the fact, that morphological examination of the brain by CT or magnetic resonance imaging (MRI) is an integral part of routine multimodal neuroprognostication approach (19), the actual patient’s management is rarely influenced (16%) and thus has a questionable impact on the patient’s outcome (7). Routine early post-resuscitation brain CT is not recommended despite being performed in a similar manner in prospective trials (20,21). In the Prague OHCA study, brain CT was requested in only 101 (39%) patients. The examinations were performed based on clinical indications, most frequently to elaborate on adverse neurological status, in patients with concomitant craniocerebral trauma, and in cases where the cause of cardiac arrest was unknown and a primary neurological origin had to be excluded.
The morphological hallmark of a hypoxic-ischemic brain injury on CT is diminished grey-to-white matter differentiation due to cytotoxic edema (19). This appearance has a high specificity (0.97) but low sensitivity (0.44) for poor neurological outcome (19,22). In our study, apparent cerebral edema equaled an unfavorable outcome, which has also been observed by others (22).
Previously reported methods for the quantification of cerebral edema on CT used absolute numbers of grey matter attenuation or more frequently GWRs (19). A GWR below 1.1 in OHCA patients is highly specific for the poor neurological outcome and the GWR cut-offs previously reported with a 100% specificity for non-survival ranged between 1.1 and 1.2 (11,23,24). In our study, we showed that these lower thresholds are acceptable, but vary across the grey matter structures measured. We additionally identified an upper threshold between 1.3 and 1.4 for GWR in deep grey matter structures. We showed that in prolonged OHCAs treated by both ECPR or conventional CPR, the GWR within a narrow range of values (1.25 to 1.35) may help to separate favorable and unfavorable outcome groups and significantly contribute to neuroprognostication within the first 36 hours after admission. Previous two studies that analyzed OHCAs treated by ECPR reported decreased GWR in patients with poor neurological outcomes with cut-off values between 1.21 and 1.24, but neither reported patients with increased GWR (11,12). Although early neuroprognostication using head CT in patients after cardiac arrest has been well documented, delaying CT may improve its predictive power (25).
Additionally, we found that patients treated with ECPR had lower attenuation of the white matter measured in the centrum semiovale. Decreased white matter attenuation in an acute setting is considered to be caused by vasogenic edema, probably due to altered cerebral autoregulation, reperfusion, hyperoxia, and non-pulsatile blood flow (26). As a denominator in the GWR equation, decreased white matter attenuation also increases the GWR.
For the quantification of the GWR, we measured attenuation in structures, that have been reported previously (17). For practical purposes, we used caudate, putamen, and thalamus that can be well detected even in patients with brain edema. The medial cortex was chosen, because there is a lower risk of partial volume bias (cortex and subarachnoid space). Centrum semiovale is a larger structure compared to the internal capsule and therefore it was preferred. In contrast to previous studies (16,17), we used oval (not circular) regions of interest of larger size to cover a greater part of the structures.
GWR has excellent specificity and PPV, but so does CT evaluation by experienced radiologists. All our patients with favorable outcome had a normal finding. This raises a question of the practical applicability and usefulness of the measurement of brain structures attenuation.
Contrast material contamination of brain CT was noted in half of the patients. All but three patients had initial coronary angiography. This is a frequent scenario in OHCAs of presumed cardiac origin as admission to cardiac centers and early invasive investigation in selected patients may bring a survival benefit (27). Because deep grey matter structures are more susceptible to hypoxic-ischemic injury, they also suffer early disruption of the blood-brain barrier. This is supported by our observation that attenuation in the thalamus and putamen correlated with attenuation in the dural sinuses probably as a result of leakage and retention of contrast material during the initial angiography, making the use of attenuation values or GWR for prognostication difficult (10).
Consequently, brain CT remains just a part of the multimodal neuroprognostication approach with numerous other predictors of patients’ outcomes combined in prognostic prediction models (28). These include patients’ demographics, prehospital circumstances, duration of cardiac arrest, cardiac rhythm, neurological criteria, laboratory values and proteomics, EEG, evoked potentials, and others (8).
Study limitations
This study has the following limitations. Firstly, brain CT was not protocolized and within the first 36 hours after cardiac arrest was performed in only 18% of all patients in the Prague OHCA study based on the clinician’s decision. Secondly, brain CT was contaminated with contrast material from the previous coronary angiography in half of the patients, again as a consequence of routine clinical triage and management of the most frequent cause of refractory OHCA.
Conclusions
Lower and upper thresholds of GWR in the putamen and caudate on early non-contrast brain CT after prolonged OHCA treated both by conventional or ECPR differentiate patients with favorable and unfavorable neurological outcomes regardless of type of CPR.
Acknowledgments
We acknowledge the work of the Prague OHCA study group who published the primary study.
Funding: This study was supported by the Ministry of Health of the Czech Republic (MH CZ-DRO, General University Hospital in Prague-VFN, No. 00064165) and by the institutional funding of the Charles University (Cooperatio, Medical Diagnostics and Basic Medical Sciences).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-430/coif). LL serves as an unpaid editorial team member of Quantitative Imaging in Medicine and Surgery. LL and AB report grant support declared in the funding statement—the Ministry of Health of the Czech Republic (MH CZ-DRO, General University Hospital in Prague-VFN, 00064165) and Charles University (Cooperatio, Medical Diagnostics and Basic Medical Sciences). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The original study as well as secondary analyses were approved by the Ethics Committee of the General University Hospital in Prague (No. 192/11 S-IV) and informed consent was taken from all individual participants or their relatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grunau B, Kime N, Leroux B, Rea T, Van Belle G, Menegazzi JJ, Kudenchuk PJ, Vaillancourt C, Morrison LJ, Elmer J, Zive DM, Le NM, Austin M, Richmond NJ, Herren H, Christenson J. Association of Intra-arrest Transport vs Continued On-Scene Resuscitation With Survival to Hospital Discharge Among Patients With Out-of-Hospital Cardiac Arrest. JAMA 2020;324:1058-67. [Crossref] [PubMed]
- Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M, et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022;327:737-47. [Crossref] [PubMed]
- Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, Collins G, Zhang L, Kalra R, Kosmopoulos M, John R, Shaffer A, Frascone RJ, Wesley K, Conterato M, Biros M, Tolar J, Aufderheide TP. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020;396:1807-16. [Crossref] [PubMed]
- Greer DM, Shemie SD, Lewis A, Torrance S, Varelas P, Goldenberg FD, et al. Determination of Brain Death/Death by Neurologic Criteria: The World Brain Death Project. JAMA 2020;324:1078-97. [Crossref] [PubMed]
- Sandroni C, Grippo A, Nolan JP. ERC-ESICM guidelines for prognostication after cardiac arrest: time for an update. Intensive Care Med 2020;46:1901-3. [Crossref] [PubMed]
- Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM, Nikolaou N, Olasveengen TM, Skrifvars MB, Taccone F, Soar J. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med 2021;47:369-421. [Crossref] [PubMed]
- Reynolds AS, Matthews E, Magid-Bernstein J, Rodriguez A, Park S, Claassen J, Agarwal S. Use of early head CT following out-of-hospital cardiopulmonary arrest. Resuscitation 2017;113:124-7. [Crossref] [PubMed]
- Coppler PJ, Flickinger KL, Darby JM, Doshi A, Guyette FX, Faro J, Callaway CW, Elmer JUniversity of Pittsburgh Post-Cardiac Arrest Service. Early risk stratification for progression to death by neurological criteria following out-of-hospital cardiac arrest. Resuscitation 2022;179:248-55. [Crossref] [PubMed]
- Geocadin RG, Callaway CW, Fink EL, Golan E, Greer DM, Ko NU, Lang E, Licht DJ, Marino BS, McNair ND, Peberdy MA, Perman SM, Sims DB, Soar J, Sandroni CAmerican Heart Association Emergency Cardiovascular Care Committee. Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation 2019;140:e517-42. [Crossref] [PubMed]
- Okimoto N, Ishida M, Abe H, Ikemura M, Fujimoto K, Kanemaru N, Ushiku T, Abe O, Gonoi W. Delayed cerebral enhancement on post-mortem computed tomography due to residual contrast medium administered shortly before death. Radiol Case Rep 2021;16:2056-60. [Crossref] [PubMed]
- Lee YH, Oh YT, Ahn HC, Kim HS, Han SJ, Lee JJ, Lee TH, Seo JY, Shin DH, Ha SO, Park SO. The prognostic value of the grey-to-white matter ratio in cardiac arrest patients treated with extracorporeal membrane oxygenation. Resuscitation 2016;99:50-5. [Crossref] [PubMed]
- Ryu JA, Chung CR, Cho YH, Sung K, Suh GY, Park TK, Song YB, Hahn JY, Choi JH, Gwon HC, Choi SH, Yang JH. The association of findings on brain computed tomography with neurologic outcomes following extracorporeal cardiopulmonary resuscitation. Crit Care 2017;21:15. [Crossref] [PubMed]
- Yang KJ, Wang CH, Huang YC, Tseng LJ, Chen YS, Yu HY. Clinical experience of whole-body computed tomography as the initial evaluation tool after extracorporeal cardiopulmonary resuscitation in patients of out-of-hospital cardiac arrest. Scand J Trauma Resusc Emerg Med 2020;28:54. [Crossref] [PubMed]
- Belohlavek J, Kucera K, Jarkovsky J, Franek O, Pokorna M, Danda J, Skripsky R, Kandrnal V, Balik M, Kunstyr J, Horak J, Smid O, Valasek J, Mrazek V, Schwarz Z, Linhart A. Hyperinvasive approach to out-of hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care. A randomized parallel groups comparative study proposal. "Prague OHCA study". J Transl Med 2012;10:163. [Crossref] [PubMed]
- Scheel M, Storm C, Gentsch A, Nee J, Luckenbach F, Ploner CJ, Leithner C. The prognostic value of gray-white-matter ratio in cardiac arrest patients treated with hypothermia. Scand J Trauma Resusc Emerg Med 2013;21:23. [Crossref] [PubMed]
- Metter RB, Rittenberger JC, Guyette FX, Callaway CW. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation 2011;82:1180-5. [Crossref] [PubMed]
- Na MK, Kim W, Lim TH, Jang B, Cho Y, Choi KS, Shin HG, Ahn C, Lee J, Kim JG. Gray matter to white matter ratio for predicting neurological outcomes in patients treated with target temperature management after cardiac arrest: A systematic review and meta-analysis. Resuscitation 2018;132:21-8. [Crossref] [PubMed]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480-4. [Crossref]
- Lopez Soto C, Dragoi L, Heyn CC, Kramer A, Pinto R, Adhikari NKJ, Scales DC. Imaging for Neuroprognostication After Cardiac Arrest: Systematic Review and Meta-analysis. Neurocrit Care 2020;32:206-16. [Crossref] [PubMed]
- Adel J, Akin M, Garcheva V, Vogel-Claussen J, Bauersachs J, Napp LC, Schäfer A. Computed-Tomography as First-line Diagnostic Procedure in Patients With Out-of-Hospital Cardiac Arrest. Front Cardiovasc Med 2022;9:799446. [Crossref] [PubMed]
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S465-82. [Crossref] [PubMed]
- Schick A, Prekker ME, Kempainen RR, Mulder M, Moore J, Evans D, Hall J, Rodin H, Larson J, Caraganis A. Association of hypoxic ischemic brain injury on early CT after out of hospital cardiac arrest with neurologic outcome. Am J Emerg Med 2022;54:257-62. [Crossref] [PubMed]
- Witten L, Gardner R, Holmberg MJ, Wiberg S, Moskowitz A, Mehta S, Grossestreuer AV, Yankama T, Donnino MW, Berg KM. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation 2019;136:93-9. [Crossref] [PubMed]
- Cristia C, Ho ML, Levy S, Andersen LW, Perman SM, Giberson T, Salciccioli JD, Saindon BZ, Cocchi MN, Donnino MW. The association between a quantitative computed tomography (CT) measurement of cerebral edema and outcomes in post-cardiac arrest-a validation study. Resuscitation 2014;85:1348-53. [Crossref] [PubMed]
- Streitberger KJ, Endisch C, Ploner CJ, Stevens R, Scheel M, Kenda M, Storm C, Leithner C. Timing of brain computed tomography and accuracy of outcome prediction after cardiac arrest. Resuscitation 2019;145:8-14. [Crossref] [PubMed]
- Wilcox C, Choi CW, Cho SM. Brain injury in extracorporeal cardiopulmonary resuscitation: translational to clinical research. J Neurocritical Care 2021;14:63-77.
- Tranberg T, Lippert FK, Christensen EF, Stengaard C, Hjort J, Lassen JF, Petersen F, Jensen JS, Bäck C, Jensen LO, Ravkilde J, Bøtker HE, Terkelsen CJ. Distance to invasive heart centre, performance of acute coronary angiography, and angioplasty and associated outcome in out-of-hospital cardiac arrest: a nationwide study. Eur Heart J 2017;38:1645-52. [Crossref] [PubMed]
- Lo YH, Siu YCA. Evaluation of prognostic prediction models for out-of-hospital cardiac arrest. Hong Kong J Emerg Med 2021;28:51-7.


