
Diffuse mass-like dural-based neurosarcoidosis with concurrent leptomeningeal involvement: an unusual case presentation and review
Introduction
Sarcoidosis is an immune-mediated non-caseating granulomatous disease that can affect numerous organ systems, including the central nervous system (CNS). Clinically, CNS involvement is found in approximately 10% of patients with sarcoidosis, but remains undiagnosed in 15–25% of this patient population (1). Isolated neurosarcoidosis (NS) is rare, with only 10–20% of patients having NS without extracranial findings (2).
Sarcoidosis is colloquially known as the great mimicker due to its highly variable clinical presentation and corresponding variable imaging manifestations (3). The preferred imaging modality to investigate NS is contrast-enhanced magnetic resonance imaging (MRI) of the brain. Thickened and nodular leptomeningeal enhancement centered in the basilar cisterns is the most common imaging finding, occurring in 40% of cases (4). Alternatively, NS may present with parenchymal, infundibular or, as in our case, pachymeningeal or dural based disease.
Case presentation
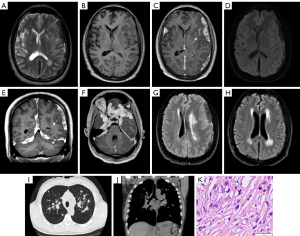
A 53-year-old male with hypertension, chronic kidney disease and a 30-pack-year smoking history presented with several months of intermittent headaches and blurry vision. He denied fever, chills, shortness of breath or cough. Initial laboratory workup revealed an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) but was otherwise unremarkable. Serology for tuberculosis, aspergillus, coccidioidomycosis, human immunodeficiency virus (HIV) and cryptococcus was negative. Lumbar puncture was attempted but was unsuccessful. MRI of the brain demonstrated multifocal extra-axial dural-based avidly enhancing mass-like lesions, as well as scattered supra- and infratentorial leptomeningeal enhancement (Figure 1). Subsequent CT of the chest revealed scattered nodules, fibrosis, and prominent mediastinal lymphadenopathy (Figure 1) for which the patient underwent endobronchial biopsy. Pathology revealed non-necrotizing granulomatous infection consistent with sarcoidosis. The dural-based lesions were not biopsied. The patient improved clinically following treatment with high-dose prednisone, along with marked reduction in disease burden on repeat MRI brain (Figure 1).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Dural based disease
Pachymeningeal NS is rare and reported to occur in 5–34% of cases of pathologically confirmed NS (5). Our patient exhibited several uncommon manifestations of pachymeningeal involvement. First, we observed diffuse extra-axial dural-based lesions along the bilateral cerebral and cerebellar convexities, tentorium and falx, an atypical pattern even in the setting of pachymeningeal involvement. Dural-based lesions more commonly present as single or scattered multifocal nodules (6). In a recent dual-center retrospective review of 100 pathologically confirmed cases of NS, 32 patients had dural involvement, 25/32 patients demonstrated single or multifocal nodularity, and only 7/32 patients demonstrated diffuse dural disease (5). Second, our patient demonstrated concomitant supra- and infratentorial involvement. Pachymeningeal NS is more often either supratentorial or infratentorial, but rarely both (7). Finally, our case demonstrated concurrent leptomeningeal involvement, an unusual finding in the setting of diffuse dural-based disease as arachnoid barrier cells typically prevent local spread between the meningeal layers (4).
Notably, while the dural-based lesions were not biopsied, the diagnosis of NS in our case was consistent with “probable NS” according to the 2018 consensus, which state that if clinical and imaging manifestations are suggestive of NS, the diagnosis may be assigned as follows: possible (no pathologic evidence); probable (pathologic confirmation outside the CNS); or definite (pathologic confirmation within the CNS) (8).
Differential diagnoses
Meningioma
Meningioma is the most common extra-axial dural-based CNS tumor. The classic imaging appearance is that of an extra-axial dural based enhancing mass with a “dural tail” sign (7). Most often, meningiomas demonstrate intermediate T1 signal, isointense T2 signal and avid homogenous enhancement following administration of gadolinium. Additionally, calcification and underlying calvarial hyperostosis may be present. These features are useful in distinguishing meningioma from dural based NS (4,7). Furthermore, meningiomas are usually isolated, though multiple tumors may be present in genetic disorders such as neurofibromatosis type 2 and meningiomatosis. Imaging features of hemangiopericytoma/solitary fibrous tumor of the dura demonstrate significant overlap with that of meningiomas (7).
Idiopathic hypertrophic pachymeningitis (IHP)
IHP is marked by chronic granulomatous inflammation leading to fibrosis. IgG4-related IHP is becoming increasingly recognized as the underlying pathology in cases previously defined as idiopathic (9). Additionally, pachymeningitis can be secondary to various etiologies, including rheumatological diseases such as rheumatoid arthritis, infections such as syphilis and tuberculosis, immunological reactions such as granulomatosis with polyangiitis, and cancer (10). Dural thickening is described by its distribution (focal or diffuse) and margins (smooth or nodular). Clinically, patients most commonly present with multiple recurrent cranial neuropathies and daily headache; an elevated ESR is often found (9,10). Given that involvement is confined to the CNS, extra-neural findings (such as mediastinal lymphadenopathy) would not be expected (9).
Dural based metastasis
Many malignancies are known to result in dural-based metastases, the most common being breast, prostate, and lung. The imaging findings of dural-based metastatic disease are similar to other pathologies including meningioma and hemangiopericytoma/solitary fibrous tumor of the dura. Metastases are usually single or multiple discrete nodules or masses but may also present as diffuse dural-based thickening. Multiplicity, the presence of concomitant osseous or parenchymal metastatic disease, and clinical history of a known primary malignancy are helpful in suggesting metastatic disease as part of a differential consideration.
Lymphoma
While primary CNS lymphoma is classically seen in immunocompromised patients and is strongly associated with HIV/acquired immune deficiency syndrome (AIDS), its incidence in immunocompromised patients, particularly those over 60 years old, is slowly increasing (11,12). Lesions can be solitary or multiple, and most commonly involve the perivascular spaces or deep gray matter. On MR imaging, lesions are T2 iso- to hypointense, with avid homogenous enhancement on post-contrast T1W sequences, and exhibit restricted diffusion (11,12). In severe cases, lesions may demonstrate central necrosis/hemorrhage, or cross the corpus callosum (11,12). In contrast to parenchymal disease observed in primary CNS lymphoma, secondary CNS lymphoma tends to present with leptomeningeal, dural, or subependymal involvement, and is associated with underlying non-Hodgkin’s lymphoma (12). Dural involvement tends to be focal, and may mimic the appearance of a meningioma, along with calvarial or transcalvarial involvement and bony erosion (11,12).
Conclusions
Although sarcoidosis is a prevalent condition, the diagnosis of NS remains difficult in part due to the wide range of imaging presentations. This was highlighted by our patient who demonstrated an unusual imaging manifestation of NS with diffuse mass-like pachymeningeal disease, concomitant leptomeningeal disease, and both supra- and infratentorial involvement. As demonstrated in our case, a firm understanding of both the common and less common radiographic features of neurosarcoidosis, as well as of the corresponding differential considerations, is required to suggest this often-elusive diagnosis. In addition, it is important to evaluate the radiographic features outside of the CNS, such as the chest CT in our case, as well as to assess clinical and laboratory findings to come to the correct diagnosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1382/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bradshaw MJ, Pawate S, Koth LL, Cho TA, Gelfand JM. Neurosarcoidosis: Pathophysiology, Diagnosis, and Treatment. Neurol Neuroimmunol Neuroinflamm 2021;8:e1084. [Crossref] [PubMed]
- Ganeshan D, Menias CO, Lubner MG, Pickhardt PJ, Sandrasegaran K, Bhalla S. Sarcoidosis from Head to Toe: What the Radiologist Needs to Know. Radiographics 2018;38:1180-200. [Crossref] [PubMed]
- Wang K, He X, Wang W, Niu H, Wang Y, Cai X, Yang S. Isolated neurosarcoidosis mimicking multifocal meningiomas: a diagnosis pitfall: A case report. Medicine (Baltimore) 2016;95:e4994. [Crossref] [PubMed]
- Smith JK, Matheus MG, Castillo M. Imaging manifestations of neurosarcoidosis. AJR Am J Roentgenol 2004;182:289-95. [Crossref] [PubMed]
- Bathla G, Freeman CW, Moritani T, Song JW, Srivastava S, Soni N, Derdeyn C, Mohan S. Retrospective, dual-centre review of imaging findings in neurosarcoidosis at presentation: prevalence and imaging sub-types. Clin Radiol 2020;75:796.e1-9. [Crossref] [PubMed]
- Savage NM, Shah H, Alleyne CH, Switzer JA, Lee JR, Steele J, Sharma S. Neurosarcoidosis with necrotising sarcoid granulomatosis mimicking meningiomatosis cerebri: case report and literature search. BMJ Case Rep 2009;2009:bcr11. [Crossref] [PubMed]
- Switlyk MD, Niehusmann P, Sprauten M, Magelssen H, Aarhus M, Rasmussen FØ, Knutstad K, Brandal P. Neurosarcoidosis resembling multiple meningiomas: A misleading presentation of the disease and diagnostic challenge. Acta Radiol Open 2021;10:20584601211036550. [Crossref] [PubMed]
- Stern BJ, Royal W 3rd, Gelfand JM, Clifford DB, Tavee J, Pawate S, Berger JR, Aksamit AJ, Krumholz A, Pardo CA, Moller DR, Judson MA, Drent M, Baughman RP. Definition and Consensus Diagnostic Criteria for Neurosarcoidosis: From the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol 2018;75:1546-53. [Crossref] [PubMed]
- Hassan KM, Deb P, Bhatoe HS. Idiopathic hypertrophic cranial pachymeningitis: Three biopsy-proven cases including one case with abdominal pseudotumor and review of the literature. Ann Indian Acad Neurol 2011;14:189-93. [Crossref] [PubMed]
- Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology 2004;62:686-94. [Crossref] [PubMed]
- Ambady P, Hu LS, Politi LS, Anzalone N, Barajas RF Jr. Primary central nervous system lymphoma: advances in MRI and PET imaging. Ann Lymphoma 2021;5:27. [Crossref] [PubMed]
- Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 2011;32:984-92. [Crossref] [PubMed]


