
Development of a predictive score for post-hemithyroidectomy hypothyroidism using skeletal muscle index, remnant thyroid index, and thyroid-stimulating hormone levels: a retrospective cohort study
Introduction
Hemithyroidectomy is a standard surgical procedure for patients with unilateral benign thyroid tumors or low-risk differentiated thyroid carcinoma (1). It is expected to be performed more often with the increase in the incidence of thyroid cancers, especially in the early stage (2).
With hemithyroidectomy, we can avoid permanent hypoparathyroidism and bilateral recurrent laryngeal nerve paralysis, which are critical complications of total thyroidectomy (3,4). Moreover, postoperative thyroid hormone replacement is unnecessary in some patients undergoing hemithyroidectomy because the hemi-lobe of the thyroid gland can be preserved. However, hypothyroidism has been reported to occur in 10.9–48.8% of post-hemithyroidectomy patients (5), and these patients require lifelong thyroid hormone replacement. Thus, hypothyroidism is considered a major complication of hemithyroidectomy. Previous studies have indicated that older patients, high preoperative thyroid-stimulating hormone (TSH) levels, lymphocytic infiltration, and Hashimoto’s thyroiditis are predictive factors for hypothyroidism after hemithyroidectomy (6-11). Recently, lower remnant thyroid volume was shown to be associated with a higher incidence of hypothyroidism after hemithyroidectomy (12,13). However, definite risk factors or clinical algorithms that can accurately predict the development of hypothyroidism after hemithyroidectomy have remained elusive.
Sarcopenia is a condition with reduced skeletal muscle mass related to aging or disease and is associated with impaired physiological reserve (14-16). Previous studies about the association of thyroid function with sarcopenia suggest that thyroid function can affect skeletal muscle mass, as thyroid hormones are engaged in muscle metabolism (17-19). One substantial role of thyroid hormones in skeletal muscle is myogenesis and regeneration (17,20,21). Hence, a lack of thyroid hormones is associated with sarcopenia. On the other hand, thyroid hormones have another function that induces the transition from a muscle fiber that can maintain muscle to the one that is involved in the sarcopenic process (20). Therefore, excessive thyroid hormone levels can also cause skeletal muscle loss. In a large cross-sectional study, the incidence of sarcopenia was higher in patients with hypothyroidism and hyperthyroidism than in those in a euthyroid state (19). Furthermore, treating patients with hyperthyroidism to achieve a euthyroid state can improve skeletal muscle mass (22). These observations indicate that skeletal muscle mass may indicate thyroid function. However, whether skeletal muscle mass is associated with thyroid function maintained by the remnant thyroid gland after hemithyroidectomy remains unknown.
This study quantitatively analyzed the preoperative skeletal muscle mass using preoperative computed tomography (CT) images to investigate the utility of skeletal muscle mass as a predictive factor for post-hemithyroidectomy hypothyroidism. Furthermore, this study evaluated previously reported risk factors, including age, preoperative TSH level, Hashimoto’s thyroiditis, and remnant thyroid volume. The association of these risk factors with postoperative thyroid function was investigated, and an attempt was made to develop a predictive score for post-hemithyroidectomy hypothyroidism using these factors. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-53/rc).
Methods
Image acquisition
This retrospective cohort study collected and retrospectively analyzed the clinical records of consecutive patients who underwent hemithyroidectomy for benign thyroid tumors or thyroid cancer at Shinshu University Hospital between January 2011 and December 2020. The inclusion criteria were as follows: (I) having undergone thyroid function tests [TSH, free triiodothyronine (FT3), and free thyroxine (FT4)] within 1 month preoperatively and 1 or 3 months postoperatively and (II) having undergone CT within 6 weeks before surgery. Patients who received preoperative levothyroxine supplementation were excluded. This study included 226 patients (Figure S1). This study conformed to the provisions of the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil; October 2013) and was approved by the Ethics Committee on Clinical Investigation of Shinshu University (No. 5036). Because this was a retrospective study of anonymized data, the informed consent requirement was waived. Our institution uses a form on its website to enable patients to opt out of the use of their clinical data for research purpose.
Data collection
Clinical information, including age, sex, height, body weight, preoperative and postoperative thyroid function (TSH, FT3, and FT4), the coexistence of Hashimoto’s thyroiditis, histological diagnosis of the resected tumor, and lymphocytic infiltration in the resected thyroid gland were collected from patients’ medical records. Hashimoto’s thyroiditis was diagnosed when either the anti-thyroglobulin antibody or anti-thyroid peroxidase antibody test was positive.
Definition of post-hemithyroidectomy hypothyroidism
Postoperative hypothyroidism was classified as subclinical, overt, or symptomatic according to a previously described definition (9). Subclinical hypothyroidism was defined as an elevated serum TSH level above the normal range (>5 µU/mL in our institute) with a normal FT4 level (≥0.9 ng/dL in our institute). Overt hypothyroidism was defined as an increase in serum TSH level (>5 µU/mL) and a decrease in serum FT4 level (<0.9 ng/dL). We defined symptomatic hypothyroidism if patients reported symptoms such as fatigue, generalized edema, weight gain, constipation, and cold intolerance with an elevated serum TSH level (>5 µU/mL). Patients with subclinical hypothyroidism were closely monitored without thyroid hormone replacement. On the other hand, for patients with overt or symptomatic hypothyroidism, thyroid hormone replacement was initiated and continued until serum TSH levels were measured within the normal range.
Evaluation of remnant thyroid volume and skeletal muscle mass
Remnant thyroid area (RTA) and skeletal muscle mass area (SMA) were measured using preoperative CT images. The estimated RTA after hemithyroidectomy was measured with the median line of the thyroid as the planned resection line in a CT image of the horizontal section through the thyroid gland by semi-automatic tracing images using the EV Insite R system (PSP Corporation, Tokyo, Japan). It was expressed in cm2 (Figure S2). SMA was measured as the area of the surrounding muscles (i.e., paraspinal, rhomboid, and trapezius) in a horizontal section image through chest CT at the fourth thoracic vertebral level visualized within a range of −29 to 150 Hounsfield units and expressed in cm2 (Figure S2). The remnant thyroid volume index (RTI) and skeletal muscle index (SMI) were calculated as the ratio of the RTA and SMA divided by the square of the patient’s height (m2), respectively. In addition, the receiver operating characteristic (ROC) curve was analyzed to determine the best SMI and RTI cutoff values for the development of hypothyroidism. The cutoff values were determined for males and females separately.
Statistical analysis
Categorical variables were analyzed using the chi-square test, whereas continuous variables were analyzed using a two-sided t-test or one-way ANOVA with Tukey’s multiple comparison for parametric analysis and Mann-Whitney U test or Kruskal-Wallis test for nonparametric analysis. Univariable and multivariable analyses with a logistic regression model were performed to determine the significant factors associated with the onset of hypothyroidism. Multivariable analysis was performed for parameters with P<0.1 in the univariable analysis. All statistical analyses were performed using Graphpad Prism 8.0.2 (Graphpad Software, San Diego, CA, USA), and P<0.05 was considered statistically significant.
Results
Patient characteristics
The clinicopathological characteristics of the 226 enrolled patients are shown in Table 1. One hundred and twenty-one patients (53.5%) were <55 years old, and 105 were ≥55 years old. One hundred and seventy-four patients (77.0%) were female, and 52 (23.0%) were male. The mean preoperative body mass index (BMI) (± standard deviation) was 23.1±3.8. Regarding preoperative thyroid function, the preoperative serum TSH, FT3, and FT4 levels (median, interquartile range) were 1.54 (1.09–2.51) µU/mL, 2.97 (2.72–3.32) pg/mL, and 1.20 (1.06–1.34) ng/mL, respectively. Sixty-three patients (27.9%) had Hashimoto’s thyroiditis. Fifty-four patients (23.9%) had benign thyroid tumors [adenomatous goiter (n=42, 18.6%), follicular adenoma (n=11, 4.9%), and hyalinizing trabecular adenoma (n=1, 0.4%)]. Meanwhile, 171 patients (75.7%) had thyroid carcinomas [papillary (n=161, 71.2%), follicular (n=3, 1.3%), medullary (n=2, 0.9%), and poorly differentiated (n=5, 2.2%) carcinomas]. In addition, one patient (0.4%) with primary hyperparathyroidism underwent hemithyroidectomy with parathyroidectomy for parathyroid carcinoma. This was due to the causal parathyroid’s large size, although no thyroid tumor was preoperatively suspected. Consequently, a histopathological examination was performed for parathyroid adenoma, and no tumors were detected in the resected thyroid gland. Lymphocytic infiltration in the resected thyroid gland was found in 41 patients (18.1%).
Table 1
| Variables | Total (n=226) | Euthyroida (n=144) | Hypothyroid | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Subclinicalb (n=50) | Overt or symptomaticc (n=32) | a vs. b | a vs. c | b vs. c | ||||
| Age, years | 0.59 | 0.83 | 0.84 | |||||
| <55 | 121 (53.5) | 84 (58.3) | 27 (54.0) | 18 (56.3) | ||||
| ≥55 | 105 (46.5) | 60 (41.7) | 23 (46.0) | 14 (43.8) | ||||
| Sex | 0.20 | 0.58 | 0.11 | |||||
| Male | 52 (23.0) | 39 (27.1) | 9 (18.0) | 4 (12.5) | ||||
| Female | 174 (77.0) | 105 (72.9) | 41 (82.0) | 28 (87.5) | ||||
| BMI, kg/m2 | 23.1±3.8 | 23.1±3.9 | 23.1±3.6 | 23.2±3.5 | 0.99 | 0.99 | 0.98 | |
| Preoperative TSH, μU/mL | 1.54 (1.09–2.51) | 1.32 (0.89–2.04) | 2.20 (1.55–3.32) | 2.30 (1.16–3.88) | <0.001 | 0.007 | 0.99 | |
| Preoperative FT3, pg/mL | 2.97 (2.72–3.32) | 3.01 (2.77–3.37) | 2.92 (2.71–3.22) | 2.84 (2.65–3.30) | 0.35 | 0.99 | 0.99 | |
| Preoperative FT4, ng/mL | 1.20 (1.06–1.34) | 1.22 (1.11–1.34) | 1.18 (1.05–1.35) | 1.02 (0.95–1.31) | 0.94 | 0.006 | 0.15 | |
| Coexistence of Hashimoto’s thyroiditis | 0.54 | 0.36 | 0.59 | |||||
| Yes | 63 (27.9) | 41 (28.5) | 12 (24.0) | 10 (31.2) | ||||
| No | 163 (72.1) | 103 (71.5) | 38 (76.0) | 22 (68.8) | ||||
| Histopathological diagnosis | 0.51 | 0.83 | 0.76 | |||||
| Benign | 54 (23.9) | 37 (25.7) | 10 (20.0) | 7 (21.9) | ||||
| Adenomatous goiter | 42 (18.6) | 29 (20.1) | 6 (12.0) | 7 (21.9) | ||||
| Follicular adenoma | 11 (4.9) | 7 (4.9) | 4 (8.0) | 0 (0.0) | ||||
| Hyalinizing trabecular adenoma | 1 (0.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||||
| Malignant | 171 (75.7) | 106 (73.6) | 40 (80.0) | 25 (78.1) | ||||
| Papillary carcinoma | 161 (71.2) | 99 (68.8) | 38 (76.0) | 24 (75.0) | ||||
| Follicular carcinoma | 3 (1.3) | 2 (1.4) | 1 (2.0) | 0 (0.0) | ||||
| Medullary carcinoma | 2 (0.9) | 1 (0.7) | 1 (2.0) | 0 (0.0) | ||||
| Poorly differentiated carcinoma | 5 (2.2) | 4 (2.8) | 0 (0.0) | 1 (3.1) | ||||
| Other | ||||||||
| Parathyroid adenoma | 1 (0.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||||
| Lymphocytic infiltration | 0.23 | 0.75 | 0.23 | |||||
| Present | 41 (18.1) | 28 (19.4) | 6 (12.0) | 7 (21.9) | ||||
| Absent | 185 (81.9) | 116 (80.6) | 44 (88.0) | 25 (78.1) | ||||
Data are presented as median (interquartile range), mean ± standard deviation or n (%). BMI, body mass index; TSH, thyroid-stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine.
Comparison of clinicopathological characteristics between the euthyroid and hypothyroid groups
Among 226 patients, postoperative hypothyroidism occurred in 82 (36.3%) (subclinical: n=50, 22.1%, overt or symptomatic: n=32, 14.2%) within 3 months after surgery. A comparison of clinicopathological characteristics between the euthyroid and hypothyroid groups is shown in Table 1. The hypothyroid group was stratified into the subclinical and the overt or symptomatic groups. No significant differences were observed in age, sex, BMI, preoperative serum FT3 and FT4 levels, the coexistence of Hashimoto’s thyroiditis, histopathological diagnosis, or lymphocytic infiltration in the euthyroid vs. subclinical hypothyroid groups, or the euthyroid vs. overt or symptomatic hypothyroid groups except for preoperative serum FT4 level between the euthyroid and overt or symptomatic hypothyroid groups. In contrast, the preoperative serum TSH level was significantly lower in the euthyroid group (median: 1.32, IQR: 0.89–2.04) than that in both the subclinical hypothyroid group (median: 2.20, IQR: 1.55–3.32, P<0.001) and the overt or symptomatic hypothyroid group (median: 2.30, IQR: 1.16–3.88, P=0.007). No significant differences were observed in any factor between the subclinical hypothyroid and overt or symptomatic hypothyroid groups.
Comparison of SMI and RTI between the euthyroid and hypothyroid groups
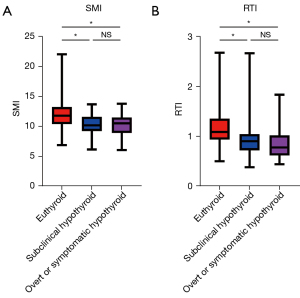
Next, SMI and RTI were compared between the euthyroid and hypothyroid groups to evaluate whether SMI or RTI was associated with the development of post-hemithyroidectomy hypothyroidism. The euthyroid group showed significantly higher SMI than that of the subclinical hypothyroid and the overt or symptomatic group (euthyroid: 11.7, 10.4–13.2 vs. subclinical hypothyroid: 10.1, 9.21–11.5, P<0.001, euthyroid vs. overt or symptomatic hypothyroid: 10.4, 9.07–11.4, P<0.001). Similarly, the RTI in the euthyroid group was significantly higher than that in the subclinical hypothyroid and the overt or symptomatic group (euthyroid: 1.08, 0.93–1.33 vs. subclinical hypothyroid: 0.89, 0.72–1.04, P<0.001, euthyroid vs. overt or symptomatic hypothyroid: 0.78, 0.62–0.99, P<0.001; Figure 1). There were no significant differences in the SMI and RTI between the subclinical hypothyroid group and the overt or symptomatic hypothyroid group.
Patients with Hashimoto’s thyroiditis are known to present with a diffuse goiter; hence, they are expected to have a higher RTI than those without this disease. Consistent with this prediction, RTI was significantly higher in the group with Hashimoto’s thyroiditis (1.05, 0.84–1.46) than in the group without non-Hashimoto’s thyroiditis (0.98, 0.78–1.19) in this cohort (P=0.04; Figure S3). The RTI between the euthyroid and hypothyroid groups in the Hashimoto’s thyroiditis and non-Hashimoto’s thyroiditis cohorts were compared to examine whether increased RTI could be found in the euthyroid group irrespective of the presence or absence of Hashimoto’s thyroiditis. A significant increase in RTI in the euthyroid group compared to that in the hypothyroid group including subclinical hypothyroid and overt or symptomatic hypothyroid was observed in both the Hashimoto’s thyroiditis (euthyroid: 1.12, 0.97–1.67, hypothyroid: 0.84, 0.68–0.99; P=0.002) and non-Hashimoto’s thyroiditis groups (euthyroid: 1.05, 0.90–1.24, hypothyroid: 0.84, 0.68–0.99; P<0.001; Figure S3). With regard to SMI, there was no significant difference in SMI between the Hashimoto’s thyroiditis (11.3, 9.96–12.2) and the non-Hashimoto’s thyroiditis groups (11.2, 10.0–12.6; P=0.42; Figure S4). However, there was a significant difference in SMI between the euthyroid and hypothyroid groups in both the Hashimoto’s thyroiditis (euthyroid: 11.7, 10.1–12.7, hypothyroid: 10.9, 9.1–11.4; P=0.002) and non-Hashimoto’s thyroiditis groups (euthyroid: 11.7, 10.5–13.4, hypothyroid: 10.2, 9.2–11.6; P<0.001; Figure S4). These results indicate that both RTI and SMI are significantly correlated with the occurrence of post-hemithyroidectomy hypothyroidism, regardless of the presence or absence of Hashimoto’s thyroiditis.
Correlation between SMI and RTI
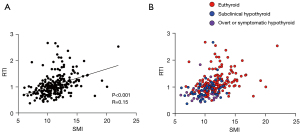
Both low SMI and RTI were associated with the incidence of hypothyroidism after hemithyroidectomy. Hence, this study sought to explore the optimal predictor of hypothyroidism using a combination of SMI and RTI. First, the correlation between SMI and RTI was examined, and a weak positive correlation was observed between both factors (R=0.15, P<0.001; Figure 2A). The scatter plot in which each patient with euthyroid status or hypothyroidism (subclinical hypothyroidism and overt or symptomatic hypothyroidism) was individually indicated (Figure 2B) suggests that patients who developed hypothyroidism were susceptible to having both low SMI and RTI.
Impact of low SMI and low RTI as a predictor for hypothyroidism after thyroidectomy
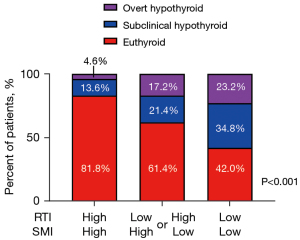
We hypothesized that patients with both low SMI and RTI are most likely to develop post-hemithyroidectomy hypothyroidism based on the results shown in Figure 2A. The patients were segregated into three groups to test this: (I) patients who had both high SMI and high RTI (SMI-high/RTI-high group, n=87); (II) those with either high SMI or high RTI (SMI-high/RTI-low or SMI-low/RTI-high group, n=70); and (III) those with both low SMI and low SMI (SMI-low/RTI-low group, n=69). The incidence of hypothyroidism was compared among these three groups. The optimal cutoff values for SMI determined by the ROC curve were 12.2 and 10.6 for males [area under the curve (AUC): 0.68] and females (AUC: 0.71), respectively. At the same time, those for RTI were 1.01 and 0.97 for males (AUC: 0.66) and females (AUC: 0.75), respectively. The incidence of overt or symptomatic hypothyroidism after thyroidectomy was lowest in the SMI-high/RTI-high group (4.6%) and gradually increased toward the SMI-high/RTI-low or SMI-low/RTI-high groups (17.2%) and the SMI-low/RTI-low group (23.2%). Similar results were obtained for subclinical hypothyroidism (SMI-high/RTI-high: 13.6%; SMI-high/RTI-low or SMI-low/RTI-high: 21.4%; SMI-low/RTI-low: 34.8%, P<0.001; Figure 3).
Univariable and multivariable analyses for post-hemithyroidectomy hypothyroidism
Univariable and multivariable analyses were performed to confirm the significance of SMI-low/RTI-low on the development of post-hemithyroidectomy hypothyroidism (subclinical hypothyroidism and overt or symptomatic hypothyroidism). The cutoff value for serum TSH levels was set at 1.65 (AUC: 0.70) according to the ROC curve. Univariable analysis revealed that SMI-low/RTI-low was significantly associated with a higher incidence of hypothyroidism [hazard ratio (HR): 3.77, 95% CI: 2.08–6.84, P<0.001]. The other factor correlated with hypothyroidism was preoperative high serum TSH levels (HR: 3.39, 95% CI: 1.92–6.00, P<0.001). Multivariable analysis showed that hypothyroidism was independently associated with SMI-low/RTI-low (HR: 3.35, 95% CI: 1.78–6.32, P<0.001) and preoperative high serum TSH levels (HR: 2.54, 95% CI: 1.39–4.65, P=0.003; Table 2).
Table 2
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| Age (<55 vs. ≥55 years) | 0.06 | 1.70 | 0.98–2.94 | 0.08 | 1.68 | 0.92–3.05 | |
| Sex (male vs. female) | 0.06 | 0.51 | 0.25–1.01 | 0.10 | 0.86 | 0.24–1.13 | |
| BMI (≥22 vs. <22 kg/m2) | 0.54 | 1.18 | 0.68–2.05 | ||||
| Preoperative serum TSH level (high vs. low) | <0.001 | 3.39 | 1.92–6.00 | 0.003 | 2.54 | 1.39–4.65 | |
| Coexistence of Hashimoto’s thyroiditis (yes vs. no) | 0.79 | 0.92 | 0.50–1.69 | ||||
| Histology (malignant vs. benign) | 0.34 | 1.37 | 0.71–2.62 | ||||
| Lymphocytic infiltration (present vs. absent) | 0.50 | 0.78 | 0.37–1.58 | ||||
| SMI-low/RTI-low (yes vs. no) | <0.001 | 3.77 | 2.08–6.84 | <0.001 | 3.35 | 1.78–6.32 | |
HR, hazard ratio; BMI, body mass index; TSH, thyroid-stimulating hormone; SMI, skeletal muscle index; RTI, remnant thyroid volume index.
Predictive score for post-hemithyroidectomy hypothyroidism
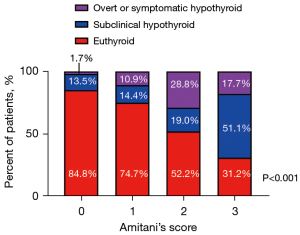
Given that high serum TSH levels and SMI-low/RTI-low were independent predictive factors for post-hemithyroidectomy hypothyroidism, an attempt was made to develop a predictive score (Amitani’s score) for post-hemithyroidectomy hypothyroidism using these three factors. A high serum TSH level, low SMI, and low RTI were each considered as one score (Table 3), and Amitani’s score was calculated by adding the score for each factor. We compared the incidence of post-hemithyroidectomy hypothyroidism between patients with Amitani’s scores of 0 (n=46), 1 (n=79), 2 (n=69), and 3 (n=32). The incidence of post-hemithyroidectomy hypothyroidism was the lowest (15.2%; subclinical: 13.5%; overt or symptomatic: 1.7%) in patients with an Amitani’s score of 0 and gradually increased in those with scores of 1 (25.3%; subclinical: 14.4%; overt or symptomatic: 10.9%) and 2 (47.8%; subclinical: 19.0%; overt or symptomatic: 28.8%). Patients with an Amitani’s score of 3 were at the highest risk for post-hemithyroidectomy hypothyroidism (68.8%; subclinical: 51.1%; overt or symptomatic: 17.7%; P<0.001; Figure 4).
Table 3
| Variables | Score | |
|---|---|---|
| 0 | 1 | |
| Serum TSH level | High (≥1.65 μU/mL) | Low (<1.65 μU/mL) |
| SMI | High (male ≥12.2, female ≥10.6) | Low (male <12.2, female <10.6) |
| RTI | High (male ≥1.01, female ≥0.97) | Low (male <1.01, female <0.97) |
TSH, thyroid-stimulating hormone; SMI, skeletal muscle index; RTI, remnant thyroid volume index.
Discussion
The results of this study suggest that reduced preoperative skeletal muscle mass is associated with the development of post-hemithyroidectomy hypothyroidism. In addition, this study demonstrated that patients with high preoperative serum TSH levels, low SMI, and low RTI are at the highest risk of post-hemithyroidectomy hypothyroidism. To the best of our knowledge, this is the first study to show that the combined assessment of serum TSH levels, skeletal muscle mass, and remnant thyroid volume can be a more accurate predictor of post-hemithyroidectomy hypothyroidism than each factor alone.
Hemithyroidectomy is generally chosen for benign tumors in the hemi-lobe or low-risk papillary thyroid carcinoma (PTC) to preserve remnant thyroid function (23). Meanwhile, active surveillance for low-risk PTC is currently considered an acceptable strategy to reduce unnecessary overtreatment (24,25). Because post-hemithyroidectomy hypothyroidism cannot be avoidable as demonstrated by the results of this study and those of previous studies (5), accurate prediction of post-hemithyroidectomy hypothyroidism before surgery may be helpful to patients and clinicians in choosing a treatment strategy. Therefore, exploring an adequate method for assessing the risk of post-hemithyroidectomy hypothyroidism is clinically important. In this regard, this study’s results showing that a condition with high preoperative serum TSH, reduced skeletal muscle, and low remnant thyroid volume is a risk factor for hypothyroidism provides novel insight into thyroid tumor treatment.
Although sarcopenia has gained much interest in cancer treatment (14), the correlation between skeletal muscle mass and thyroid volume or function has not yet been demonstrated. Regarding the association between thyroid volume and patient physique, it has been shown that thyroid volume positively correlates with BMI (26). Consistent with this report, a positive correlation between BMI and RTI was found in this study’s patient cohort (data not shown). However, regarding thyroid function after hemithyroidectomy, BMI did not correlate with the incidence of hypothyroidism, as shown in Table 1 and Table 2. The present study demonstrated that SMI was significantly lower in patients who developed post-hemithyroidectomy hypothyroidism than in those who maintained euthyroid status. These findings indicate that SMI is superior to BMI in evaluating thyroid function reserve. Although the proportions of body composition, such as visceral fat, subcutaneous fat, and skeletal muscle, vary among individuals (27), BMI cannot discriminate between adipose tissue and muscles. On the other hand, SMI can accurately evaluate skeletal muscle mass per patient’s physique. The advantage of SMI in evaluating skeletal muscle mass would contribute, at least in part, to the higher potential of SMI than that of BMI in predicting the incidence of post-hemithyroidectomy hypothyroidism. However, the precise mechanisms underlying the association between SMI and thyroid function reserves remain unclear. Hence, further studies are required to clarify this point.
Regarding remnant thyroid volume, a previous study demonstrated a significant inverse association between the preoperative contralateral lobe volume and the risk of post-hemithyroidectomy hypothyroidism (13). Their study measured the remnant thyroid volume using ultrasonographic volumetry (13). However, the ultrasonography measurement tends to be operator-dependent, and operator bias is a major concern for this type of evaluation. In comparison, measurements using CT images enable a more objective evaluation of the thyroid volume. In addition, volume measurement using CT images is considered a standard method for evaluating skeletal muscle mass (28,29). The present study utilized CT images to estimate the remnant thyroid volume and showed that a small volume of the contralateral lobe was significantly associated with a high incidence of post-hemithyroidectomy hypothyroidism. To evaluate RTA, CT images recorded postoperatively would be ideal rather than those recorded preoperatively. However, postoperative CT images were not available for most of the patients in our cohort. One reason was that postoperative CT was not performed for patients with benign tumors (23.9% in our cohort). Furthermore, even for patients with thyroid cancer, follow-up CT was not routinely performed unless the serum thyroglobulin level was elevated. Hence, we used the preoperative CT images to estimate RTA. Although CT examination may not be performed preoperatively for all patients undergoing thyroid surgery, particularly for those with benign tumors, CT would be a useful modality to evaluate thyroid volume, if applicable.
Of the previously reported risk factors for hypothyroidism, including high preoperative TSH, older age, lymphocytic infiltration, and coexistence of Hashimoto’s disease (6-11), high preoperative TSH was a predictor in the present study. However, being older, the presence of lymphocytic infiltration, and the coexistence of Hashimoto’s disease were not significantly associated with the incidence of hypothyroidism in this study. Regarding the age of patients, the lack of association between old age and hypothyroidism might be due to the small number of patients included in this study (α error) because a marginal significance was observed in the univariable and multivariable analyses. On the other hand, the present study showed that the presence of lymphocytic infiltration and the coexistence of Hashimoto’s disease had little influence on the development of hypothyroidism, which was contradictory to a previous study (8,12). One possible explanation for this discordant result is that this study’s follow-up period was shorter than that of the previous studies. The follow-up period in this study was within 3 months postoperatively, whereas the median follow-up period of the previous studies ranged from 22 to 69 months (8,12). Thus, a longer follow-up period might have revealed the impact of lymphocytic infiltration or Hashimoto’s disease on this study’s cohort.
This study had several limitations. First, this was a retrospective analysis with a relatively small study population. Second, this study was conducted at a single institution in an Asian country with adequate iodine intake. Considering that it is generally accepted that thyroid volume and function are affected by iodine intake (30), this study’s results cannot be conclusively applied to patients in iodine-intake-deficient regions. Third, the follow-up period was short (3 months postoperatively). A large-scale prospective multi-regional study with long-term follow-up is required to overcome these limitations and confirm this study’s findings.
Conclusions
The results of the present study indicate that a combination of high serum TSH levels, reduced skeletal muscle mass, and low remnant thyroid volume would be useful in predicting the development of post-hemithyroidectomy hypothyroidism. Hence, reproducing this observation in more extensive studies might further highlight the importance of evaluating preoperative serum TSH levels, skeletal muscle mass, and remnant thyroid volume to predict post-hemithyroidectomy hypothyroidism.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-53/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-53/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The design of this study was approved by the Ethics Committee on Clinical Investigation of Shinshu University (No. 5036) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All data were retrieved retrospectively and used in an anonymized fashion. The requirement for informed consent from patients was waived because of its retrospective design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Dedivitis RA, Aires FT, Cernea CR. Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg 2017;25:142-6. [Crossref] [PubMed]
- Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687-95. [Crossref] [PubMed]
- Kandil E, Krishnan B, Noureldine SI, Yao L, Tufano RP. Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL J Otorhinolaryngol Relat Spec 2013;75:6-17. [Crossref] [PubMed]
- Chu KK, Lang BH. Clinicopathologic predictors for early and late biochemical hypothyroidism after hemithyroidectomy. Am J Surg 2012;203:461-6. [Crossref] [PubMed]
- Johner A, Griffith OL, Walker B, Wood L, Piper H, Wilkins G, Baliski C, Jones SJ, Wiseman SM. Detection and management of hypothyroidism following thyroid lobectomy: evaluation of a clinical algorithm. Ann Surg Oncol 2011;18:2548-54. [Crossref] [PubMed]
- Koh YW, Lee SW, Choi EC, Lee JD, Mok JO, Kim HK, Koh ES, Lee JY, Kim SC. Prediction of hypothyroidism after hemithyroidectomy: a biochemical and pathological analysis. Eur Arch Otorhinolaryngol 2008;265:453-7. [Crossref] [PubMed]
- Lee SJ, Song CM, Ji YB, Choi YY, Sohn YS, Park JH, Kim DS, Tae K. Risk factors for hypothyroidism and thyroid hormone replacement after hemithyroidectomy in papillary thyroid carcinoma. Langenbecks Arch Surg 2021;406:1223-31. [Crossref] [PubMed]
- Moon HG, Jung EJ, Park ST, Jung TS, Jeong CY, Ju YT, Lee YJ, Hong SC, Choi SK, Ha WS. Thyrotropin level and thyroid volume for prediction of hypothyroidism following hemithyroidectomy in an Asian patient cohort. World J Surg 2008;32:2503-8. [Crossref] [PubMed]
- Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab 2012;97:2243-55. [Crossref] [PubMed]
- Ahn D, Lee GJ, Sohn JH. Levothyroxine Supplementation Following Hemithyroidectomy: Incidence, Risk Factors, and Characteristics. Ann Surg Oncol 2019;26:4405-13. [Crossref] [PubMed]
- Lang BH, Wong CKH, Wong KP, Chu KK, Shek TWH. Effect of Thyroid Remnant Volume on the Risk of Hypothyroidism After Hemithyroidectomy: A Prospective Study. Ann Surg Oncol 2017;24:1525-32. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Karakousis ND, Chrysavgis L, Chatzigeorgiou A, Papatheodoridis G, Cholongitas E. Frailty in metabolic syndrome, focusing on nonalcoholic fatty liver disease. Ann Gastroenterol 2022;35:234-42. [Crossref] [PubMed]
- Lynch DH, Spangler HB, Franz JR, Krupenevich RL, Kim H, Nissman D, Zhang J, Li YY, Sumner S, Batsis JA. Multimodal Diagnostic Approaches to Advance Precision Medicine in Sarcopenia and Frailty. Nutrients 2022;14:1384. [Crossref] [PubMed]
- Bloise FF, Oliveira TS, Cordeiro A, Ortiga-Carvalho TM. Thyroid Hormones Play Role in Sarcopenia and Myopathies. Front Physiol 2018;9:560. [Crossref] [PubMed]
- Sheng Y, Ma D, Zhou Q, Wang L, Sun M, Wang S, Qi H, Liu J, Ding G, Duan Y. Association of thyroid function with sarcopenia in elderly Chinese euthyroid subjects. Aging Clin Exp Res 2019;31:1113-20. [Crossref] [PubMed]
- Szlejf C, Suemoto CK, Janovsky CCPS, Barreto SM, Diniz MFHS, Lotufo PA, Bensenor IM. Thyroid Function and Sarcopenia: Results from the ELSA-Brasil Study. J Am Geriatr Soc 2020;68:1545-53. [Crossref] [PubMed]
- Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. Thyroid hormones and skeletal muscle--new insights and potential implications. Nat Rev Endocrinol 2014;10:206-14. [Crossref] [PubMed]
- Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 2008;18:205-16. [Crossref] [PubMed]
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006;16:375-80. [Crossref] [PubMed]
- Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw 2018;16:1429-40. [Crossref] [PubMed]
- Ahmadi S, Alexander EK. Active Surveillance for Low-Risk Differentiated Thyroid Cancer. Endocr Pract 2023;29:148-53. [Crossref] [PubMed]
- Ho AS, Bastien AJ, Sacks WL. Thyroid Cancer Active Surveillance: The Devil You Know or The Devil You Don't. Thyroid 2022;32:1279-80. [Crossref] [PubMed]
- Xiao Y, Mao J, Mao X, Wang Q, Li X, Chen G, Guo L, Huang H, Mu Y, Xu S, Liu C. Metabolic syndrome and its components are associated with thyroid volume in adolescents. BMC Endocr Disord 2021;21:176. [Crossref] [PubMed]
- Davis MP, Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann Palliat Med 2019;8:86-101. [Crossref] [PubMed]
- Blauwhoff-Buskermolen S, Versteeg KS. de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, Verheul HM. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol 2016;34:1339-44. [Crossref] [PubMed]
- Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, Quesenberry CP, Weltzien EK, Castillo AL, Olobatuyi TA, Chen WY. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 2018;4:798-804. [Crossref] [PubMed]
- Melse-Boonstra A, Mackenzie I. Iodine deficiency, thyroid function and hearing deficit: a review. Nutr Res Rev 2013;26:110-7. [Crossref] [PubMed]


