
18F-FDG PET/CT findings of hepatic and lung epithelioid hemangioendothelioma
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare form of low-grade malignant stromal tumor with metastatic potential that lies intermediately between hemangiomas and angiosarcomas (1-3). EHE mainly occurs in superficial and deep soft tissues but can also occur in solid organs such as the liver, lungs, bone, brain, small intestine, and spleen (4). Due to its atypical clinical symptoms, EHE can easily be misdiagnosed as metastases, epithelioid hemangiopericytoma, cholangiocarcinoma, and so on (5,6). Very little is known about EHE with regards to positron emission tomography (PET) imaging.
Case presentation
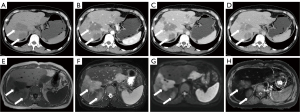
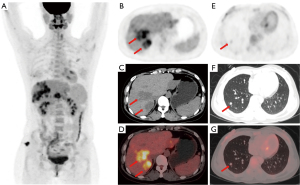
A 35-year-old woman underwent laparoscopic pelvic lesion resection for pelvic endometriosis. Routine follow-up computed tomography (CT) revealed a fusiform mass at the right border of the liver. Further contrast-enhanced CT showed that the liver margin was less smooth with large low-density lesions; the possibility of confluent fibrosis in cirrhosis was considered. In addition, contrast-enhanced magnetic resonance imaging (MRI) revealed multiple flaky, nodular, hypo-enhanced foci in the liver with restricted diffusion along with some foci adjacent to the shrunken hepatic capsule (Figure 1). Considering the presence of malignant tumor lesions, the possibility of metastasis was considered. Since the onset of the disease, the patient had experienced reduced vitality and quality of sleep, normal urination and defecation, no change in body weight, and no other particular discomfort. The results of laboratory tests for liver function and tumor markers were normal. To rule out the possibility of metastases, we performed 18F-fluorodeoxyglucose (18F-FDG) PET/CT. Imaging revealed multiple low-density foci with moderate hypermetabolism in the liver parenchyma, and multiple solid nodules with mild hypermetabolism in both lungs (Figure 2). Liver and lung biopsies were performed, and according to the results of pathology and immunohistochemistry, the patient was diagnosed with EHE. Later, the patient was discharged without any unusual discomfort and was advised to receive treatment in a specialized liver hospital. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
18F-FDG PET/CT manifestations vary for different types of liver disease. In hepatocellular carcinoma (HCC), the level of glucose metabolism is related to the degree of tumor differentiation. Poorly differentiated HCC exhibits high uptake of 18F-FDG, whereas the 18F-FDG uptake of well-differentiated HCC is close to or even slightly lower than that of normal liver parenchyma (7,8). Cholangiocarcinoma presents as an intrahepatic low-density lesion with abnormal elevated glucose metabolism, uneven density, unclear boundary, an irregularly dilated intrahepatic bile duct, and local hepatic lobe atrophy (9). Metastatic liver cancer is usually characterized by single or multiple lesions with abnormal elevated glucose metabolism in the liver (10). 18F-FDG PET/CT images of hepatic abscesses show a ring with high 18F-FDG uptake and a low-density lesion with blurred edges. The advantage of 18F-FDG PET/CT in the diagnosis of liver tumors lies in the comprehensive and accurate staging, restaging, and efficacy evaluation. The downside is that it can lead to false negative results when the tumor is too small or well differentiated (11,12).
In such cases, 18F-FDG PET/CT as a systemic examination can provide a comprehensive assessment of the lesion. When a poorly defined, low-density hepatic mass with moderate hypermetabolism is observed, hepatic EHE should be included in the differential diagnosis for liver cancer.
Acknowledgments
Funding: This work was supported by grants from Shenzhen Science Technology Project (No. JCYJ20210324114005015 to Y.Y.) and Guangdong Province Basic and Applied Basic Research Foundation (No. 2021A1515220068 to Y.Y.).
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1422/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Witte S, Weidema M, Kaal S, Versleijen-Jonkers Y, Flucke U, van der Graaf W, Desar I. The heterogeneity of Epithelioid Hemangioendothelioma (EHE): A case series and review of the literature with emphasis on treatment options. Semin Oncol 2021;48:111-8. [Crossref] [PubMed]
- Mao X, Liang Z, Chibhabha F, Ou W, Li N, Xu P, Wang S. Clinico-radiological features and next generation sequencing of pulmonary epithelioid hemangioendothelioma: A case report and review of literature. Thorac Cancer 2017;8:687-92. [Crossref] [PubMed]
- Kou K, Chen YG, Zhou JP, Sun XD, Sun DW, Li SX, Lv GY. Hepatic epithelioid hemangioendothelioma: Update on diagnosis and therapy. World J Clin Cases 2020;8:3978-87. [Crossref] [PubMed]
- Jang JK, Thomas R, Braschi-Amirfarzan M, Jagannathan JP. A Review of the Spectrum of Imaging Manifestations of Epithelioid Hemangioendothelioma. AJR Am J Roentgenol 2020;215:1290-8. [Crossref] [PubMed]
- Rosenberg A, Agulnik M. Epithelioid Hemangioendothelioma: Update on Diagnosis and Treatment. Curr Treat Options Oncol 2018;19:19. [Crossref] [PubMed]
- Studer LL, Selby DM. Hepatic Epithelioid Hemangioendothelioma. Arch Pathol Lab Med 2018;142:263-7. [Crossref] [PubMed]
- He YX, Guo QY. Clinical applications and advances of positron emission tomography with fluorine-18-fluorodeoxyglucose (18F-FDG) in the diagnosis of liver neoplasms. Postgrad Med J 2008;84:246-51. [Crossref] [PubMed]
- Liao X, Wei J, Li Y, Zhong J, Liu Z, Liao S, Li Q, Wei C. 18F-FDG PET with or without CT in the diagnosis of extrahepatic metastases or local residual/recurrent hepatocellular carcinoma. Medicine (Baltimore) 2018;97:e11970. [Crossref] [PubMed]
- Notaristefano A, Niccoli Asabella A, Stabile Ianora AA, Merenda N, Moschetta M, Antonica F, Altini C, Ferrari C, Cesarano E, Rubini G. 18F-FDG PET/CT in staging and restaging cholangiocarcinoma. Recenti Prog Med 2013;104:328-35. [PubMed]
- Sun L, Wu H, Guan YS. Positron emission tomography/computer tomography: challenge to conventional imaging modalities in evaluating primary and metastatic liver malignancies. World J Gastroenterol 2007;13:2775-83. [Crossref] [PubMed]
- Lee SM, Kim HS, Lee S, Lee JW. Emerging role of (18)F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World J Gastroenterol 2019;25:1289-306. [Crossref] [PubMed]
- Kornberg A, Friess H. (18)F-fludeoxyglucose positron emission tomography for diagnosis of HCC: implications for therapeutic strategy in curative and non-curative approaches. Therap Adv Gastroenterol 2019;12:1756284819836205. [Crossref] [PubMed]


