
Upper trapezius muscle elasticity in cervical myofascial pain syndrome measured using real-time ultrasound shear-wave elastography
Introduction
Neck pain is a common condition (prevalence of 20%) that causes long-lasting disability (ranked fourth in terms of number of years). Its lifetime prevalence is approaching 70%, and the rate of recurrence is high (1,2). It therefore represents a serious global public health problem (1,2). Myofascial pain syndrome (MPS) is a common cause of chronic neck pain (1,3-6). Cervical MPS is caused by neck muscle strain, and manifests as long-term neck pain, stiffness, and dysfunction caused by myofascial trigger points (MTrPs) in skeletal muscle (1,6-9). Because pain is subjective, MPS has been ignored in the past and has been considered less serious than other conditions causing disability. However, patients with MTrPs report pain severity scores on the visual analog scale (VAS) that are equivalent to, or higher than, those reported by patients with pain from other causes traditionally considered serious, such as angina and bone fractures (6). Furthermore, pain can seriously affect quality of life (1,6). Despite its seriousness, pain from MTrPs is still diagnosed on the basis of purely clinical parameters: at this stage, there are no known morphological changes at the level of muscle tissue, nor positive findings from conventional imaging or laboratory testing. Diagnosis relies primarily on expert palpation of MTrPs, physical examination, and patient symptoms, which have poor reliability (10). Additionally, the chief complaint of “pain” in MPS patients is a subjective experience and is susceptible to psychological, social, and environmental factors (1,6). Various pain assessment methods (such as the VAS) are currently available, however they all require patient compliance with the assigned instructions, and rely on patient ability to communicate (10). Therefore, patients with communication disorders are difficult to assess (10-12). The lack of objective and reliable diagnostic MPS indicators represents a challenging clinical problem (6,13-15).
According to the European Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications (2018 update), skeletal muscle diseases can alter the elastic properties of muscle tissue (16). As a consequence, the evaluation of changes in muscle elasticity carries significant potential for diagnosis and evaluation of skeletal muscle diseases. Real-time ultrasound shear-wave elastography can locate and detect tissue elasticity. This technology is based on the superposition of shear-wave tissue elastic imaging data onto two-dimensional ultrasound images. Currently, it is predominantly used for the evaluation of benign and malignant lesions in the thyroid, breast, and liver (16-19). Only a few studies have examined its use for skeletal muscle lesions, and they have focused mainly on the evaluation of muscle or tendon stiffness in various neurological diseases and Achilles tendon injuries (20-26). Previous cervical MPS studies have focused on assessing elasticity changes at the level of cervical skeletal muscles and MTrPs within the trapezius. Cervical MPS patients reportedly exhibit significantly higher trapezius muscle stiffness (27-30). However, the value of this and other changes for diagnosing MPS remains unclear. In particular, it is not known how changes in trapezius muscle elasticity relate to pain severity in MPS patients. To address this issue, our study aimed to evaluate elasticity changes in the upper trapezius muscle in cervical MPS patients using real-time ultrasonic shear wave elastography (SWE), and determine their value in diagnosing MPS and evaluating pain severity. We hypothesized that cervical MPS patients are affected by enhanced stiffness of trapezius muscles, and that the associated elasticity changes carry valuable information for diagnosing and assessing pain severity. This article is presented in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-797/rc).
Methods
Subjects
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved (No. YXLL-2020-030) by the ethics review committee of the Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital). All participants provided written informed consent. This is a prospective study for which all participants were recruited in a consecutive manner. We identified 293 eligible patients with posterior cervical pain treated at our institution between November 2020 and May 2021 (Figure 1). Inclusion criteria were: aged 20–45 years, body mass index (BMI) of 18.5–23.9 kg/m2, right-handed, and a chief complaint of posterior neck pain. Exclusion criteria were: neck pain treatment prior to the ultrasound examination; history of shoulder or neck trauma, surgery, or radiotherapy (n=12); history of primary muscle disease (n=4); and history of rheumatic disease, diabetes, hypertension, malignancy, psychosis, thyroid disease, or ≥2 migraine headaches per month (n=81). We also excluded patients with communication disorders and those who were unable to cooperate with the pain assessment procedure.
Patients were assigned to either MPS or non-MPS group based on the presence or absence of cervical MPS. This condition was diagnosed by two clinicians with more than 10 years of experience using the criteria proposed by Simons et al., which are widely adopted for clinical diagnosis of MPS (6). The major criteria include: (I) palpable taut band (if muscle is accessible); (II) exquisite spot tenderness of a nodule in a taut band; (III) patient report of concurrent pain following pressure on the tender nodule (for active MTrPs); (IV) painful limit to full stretch range of motion (6). All subjects in the MPS group met these diagnostic criteria, while those in the non-MPS group did not. For the latter patients, it was always possible to reliably identify an alternative origin for their neck pain via relevant imaging examinations. Any disagreement in diagnosis was resolved by a third clinician with more than 10 years of clinical experience. The overall rate of diagnostic agreement between the two clinicians was 96.49% (110/114). Right neck pain in both MPS and non-MPS patients was scored using the VAS (10-12), in which participants scored their pain levels on a scale between 0 to 10. A VAS score of 1–3 was graded as mild, a score of 4–6 was graded as moderate, and a score of 7–10 was graded as severe. Evaluators were blinded to the elasticity assessments.
Following the procedures detailed above, we enrolled a total of 114 patients: 54 patients with MPS in the right side of the neck, and 60 patients with non-MPS in the right side of the neck.
Image acquisition and analysis of upper trapezius muscle elasticity
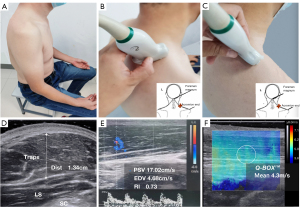
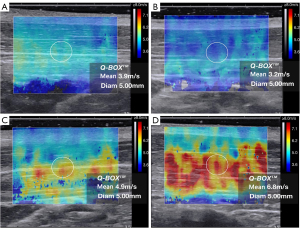
After enrollment, all subjects underwent ultrasonography of the trapezius before receiving neck pain treatment. Ultrasonography was performed by one of two experienced sonographers who were blind to patient grouping and other clinical data. Room temperature was maintained at 24 ℃ during the procedure. An L10-2 linear array probe with a frequency of 2 to 10 MHz was used for real-time ultrasound shear-wave elastic imaging (AixPlorer, Supersonic Imagine, Aix-en-Provence, France). All subjects were seated with their shoulders and neck fully exposed and forearms relaxed, with palms facing up on the ipsilateral thigh (Figure 2A). The midpoint of the line between the foramen magnum and the end of the right acromion was selected as the measuring point on the trapezius muscle. The probe was placed at the measurement point and directed perpendicularly to the long axis of the trapezius to obtain a transverse image (Figure 2B). Muscle thickness and cross-sectional area were measured. The probe was then rotated 90 degrees counterclockwise (parallel to the long axis of the trapezius) to obtain a longitudinal image (Figure 2C). Peak systolic velocity (PSV), end-diastolic velocity (EDV), and the resistance index (RI) were measured using Doppler ultrasonography. The RI was calculated using the formula (PSV − EDV)/PSV. This ratio helps to differentiate between various vascular abnormalities (27,31). Muscle thickness of the trapezius was measured from a cross-sectional image of the trapezius muscle (Figure 2D). PSV, EDV, and RI were measured from a longitudinal section of the trapezius muscle (Figure 2E). For shear-wave elastography, the probe direction was parallel to the long axis of the trapezius muscle, and a region of interest (ROI) was placed in the middle of the image to cover the trapezius muscle belly and surrounding tissue. The ROI was a 2.0 cm × 3.0 cm square, with the scale set to span 0–8.0 m/s. The numbers 0–8.0 m/s indicate different degrees of elasticity: 0 m/s indicates soft tissue and low stiffness, which is represented by blue coloring in Figure 2F, while 8.0 m/s indicates hard and stiff tissue, which is represented by red coloring in Figure 2F. A blue to red gradient thus indicates a gradual increase in tissue stiffness (Figure 2F), with yellow corresponding to moderate stiffness. Therefore, trapezius stiffness can be gauged from the overall color distribution of the SWE image. The probe was placed perpendicularly to the skin surface, and slight pressure was applied (21). After the image was stabilized, it was frozen and submitted to the quantitative analysis system (Q-BOX). Q-BOX was set to a circle with a 5-mm diameter centered on the trapezius muscle (the center of the image). The system automatically calculated the mean shear-wave propagation velocity (SWVmean, unit: m/s) (32,33). We averaged SWVmean over three repeated measurements.
Cervical MPS was the adjudicated final diagnosis in 54 patients; however, three of these patients were excluded (two were unable to relax their shoulder and neck to cooperate with the imaging, and image transmission was incomplete in one patient). Therefore, 51 MPS patients were finally included in the study. We enrolled 60 non-cervical MPS patients in the study. Of these, two were excluded (one was unable to relax their shoulder and neck to cooperate with the imaging procedure, and one declined to participate). Therefore, 58 control patients were included in the study (Figure 1).
Intra- and interobserver reproducibility
To assess intra-observer reproducibility, the same sonographer repeated ultrasonic elasticity image acquisition and analysis in 10 randomly selected subjects, with 5 days of interval between patients and in random order. We also assessed inter-observer reproducibility from the same subjects. Both were evaluated using Bland-Altman plots and the intraclass correlation coefficient (ICC). Reproducibility was categorized as follows: good for ICC ≥0.75, moderate for 0.4≤ ICC <0.75, and poor for ICC <0.4.
Sample size estimation
Based on preliminary tests involving 20 subjects (12 MPS patients and 8 non-MPS patients), we estimated that an area under the curve (AUC) value of 0.74 would be adequate for diagnosing elasticity changes in the upper trapezius. Sample size was calculated using the single diagnostic test receiver operating characteristic (ROC) curve method in the PASS software (PASS2021 for Windows, NCSS, LLC., Kaysville, Utah, USA) (β≤0.1, 90% degree of grasp [power =1−β], and a bilateral significance level of α=0.05). The calculated sample size for each group was 37. Assuming a 10% dropout rate, we required a minimum of 41 patients for each group.
Statistical analysis
All data were analyzed using the SPSS statistical software (SPSS for Windows, version 22.0; IBM, Armonk, NY, USA) and the MedCalc software (MedCalc for Windows, version 19.6.4; MedCalc Software, Mariakerke, Belgium). We used the Shapiro-Wilk test to assess normality of data distribution, and the Levene’s test to analyze homogeneity of variance. Normally distributed data are expressed as means with standard deviation. Data with a skewed distribution are expressed as medians with interquartile range. We used independent samples t-tests or Mann-Whitney U tests to analyze group differences in thickness, cross-sectional area, RI, and SWVmean. We used one-way analyses of variance and Kruskal-Wallis tests to analyze differences in SWVmean between control and patient groups stratified based on pain severity. Spearman’s rank correlation was used to analyze the correlation between SWVmean and VAS score. ROC analysis was used to evaluate the diagnostic performance of SWVmean for cervical MPS. The maximum Youden index was selected as the optimal cut-off value, and sensitivity, specificity, and the 95% confidence interval (CI) of the optimal cut-off value were calculated. Statistical testing was two-sided, and a P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 109 patients were enrolled in this study. Of these, 51 patients were diagnosed with right cervical MPS [mean age 30.15±6.04 years (range, 20.00–44.00 years); mean BMI 22.17±1.52 kg/m2 (range, 18.69–23.89 kg/m2); 25 women and 26 men], and 58 were non-cervical MPS patients [mean age 32.20±6.25 years (range, 20.00–44.00 years); mean BMI 21.84±1.41kg/m2 (range, 18.47–23.83 kg/m2); 30 women and 28 men]. Age, sex, and BMI did not significantly differ between MPS and non-MPS patients (Table 1).
Table 1
| Variables | MPS patients (n=51) | Non-MPS patients (n=58) | P |
|---|---|---|---|
| Men/women | 26/25 | 28/30 | 0.778 |
| Age (years) | 30.15±6.04 (20.00–44.00) | 32.20±6.25 (20.00–44.00) | 0.085 |
| BMI (kg/m2) | 22.17±1.52 (18.69–23.89) | 21.84±1.41 (18.47–23.83) | 0.250 |
| Clinical diagnosis | 0.005* | ||
| Cervical spondylosis | 34 (66.67) | 23 (39.66) | |
| Other | 6 (11.76) | 35 (60.34) | |
| Pain on the right/bilateral | 17/34 | 13/45 | 0.203 |
| VAS score of the right neck | 0.434 | ||
| Mild pain (1–3 scores) | 19 (37.25) | 15 (25.86) | |
| Moderate pain (4–6 scores) | 25 (49.02) | 32 (55.17) | |
| Severe pain (7–10 scores) | 7 (13.73) | 11 (18.97) |
Values are shown as means ± standard deviation or numbers (percentage). Range for age and BMI are shown in parentheses. *, statistically significant (P<0.05). MPS, myofascial pain syndrome; BMI, body mass index; VAS, visual analogue scale.
Among MPS patients, 11 did not present complications from other cervical spine diseases (21.57%), while the remaining 40 patients (78.43%) suffered from other cervical diseases: 34 (66.67%) had cervical spondylosis, and 6 had other diseases (11.76%), including three patients with scapulohumeral periarthritis and three patients with atlantoaxial subluxation. Non-MPS patients with other causes of right-sided neck pain included 23 patients with cervical spondylosis (39.66%) and 35 patients with other diseases (60.34%). Other diseases included eight cases of scapulohumeral periarthritis, six cases of cervical radiculitis, six cases of atlantoaxial subluxation, five cases of cervical tuberculosis, four cases of thoracic outlet syndrome, three cases of cervical tumor, two cases of postherpetic neuralgia, and one case of syringomyelia (Table 1).
Among MPS patients, 19 (37.25%), 25 (49.02%), and 7 (13.73%) patients returned VAS pain scores of 1–3 (mild), 4–6 (moderate), and 7–10 (severe), respectively. Corresponding figures for non-MPS patients across the same three score ranges were 15 (25.86%), 32 (55.17%), and 11 patients (18.97%). Pain severity did not significantly differ between MPS and non-MPS patients (P=0.434) (Table 1).
Inter- and intraobserver reproducibility
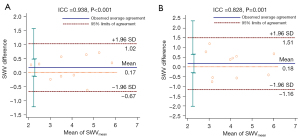
Bland-Altman agreement plots for trapezius SWVmean are shown in Figure 3A for one observer, and in Figure 3B for two observers. ICC values for intra- and interobserver reproducibility were 0.938 (95% CI: 0.773–0.984; P<0.001) and 0.828 (95% CI: 0.450–0.954; P=0.001), respectively, indicating good reproducibility for both.
Conventional ultrasound
Trapezius muscle thickness and RI did not significantly differ between MPS and non-MPS patients (P=0.445, P=0.119; Table 2).
Table 2
| Variables | MPS patients (n=51) | Non-MPS patients (n=58) | P |
|---|---|---|---|
| Thickness (cm) | 1.28±0.20 (0.93–1.86) | 1.25±0.25 (0.78–1.88) | 0.445 |
| RI | 0.67±0.09 (0.52–0.83) | 0.65±0.10 (0.44–0.80) | 0.119 |
Values are shown as mean ± standard deviation (range). MPS, myofascial pain syndrome; RI, resistance index
Elastography
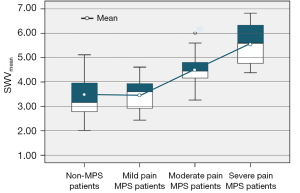
SWVmean values for the trapezius were significantly higher in MPS patients compared with non-MPS patients (4.30±0.95 versus 3.33±0.69 m/s; P<0.001; Table 3). In stratified analysis of MPS patients based on pain severity, trapezius SWVmean values did not significantly differ between the mild pain MPS and non-MPS patients (3.51±0.59 versus 3.33±0.69 m/s; P=0.324); however, they significantly differed between moderate pain MPS and non-MPS patients (4.58±0.73 versus 3.33±0.69 m/s; P<0.001) and between severe pain MPS and non-MPS patients (5.40±0.80 versus 3.33±0.69 m/s; P<0.001; Table 3 and Figure 4).
Table 3
| Groups | Number of patients (%) | SWVmean (m/s) | P | ||
|---|---|---|---|---|---|
| Mean ± standard deviation | Range | Mean difference (95% CI) | |||
| Non-MPS patients | 58 | 3.33±0.69 | 2.00–5.12 | – | – |
| MPS patients | 51 | 4.30±0.95 | 2.44–6.83 | 0.96 (0.65–1.28) | <0.001 |
| Mild pain | 19 (37.25) | 3.51±0.59 | 2.44–4.60 | 0.18 (−0.18 to 0.53) | 0.324 |
| Moderate pain | 25 (49.02) | 4.58±0.73 | 3.46–6.45 | 1.25 (0.92–1.59) | <0.001 |
| Severe pain | 7 (13.73) | 5.40±0.80 | 4.37–6.83 | 2.07 (1.51–2.63) | <0.001 |
Values are shown as mean ± standard deviation, 95% confidence intervals or percentages. SWVmean, mean shear-wave velocity; CI, confidence interval; MPS, myofascial pain syndrome.
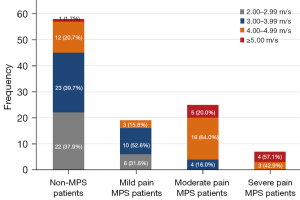
SWVmean values in non-MPS and mild pain MPS patients mainly fell below 4.00 m/s and accounted for 77.59% (45/58) and 84.21% (16/19) of patients, respectively. The real-time ultrasound shear-wave elastography images of the trapezius muscle for these groups were predominantly blue-green. The proportions of moderate pain MPS patients with SWVmean values below 4.00 m/s and above 4.00 m/s were 16% (4/25) and 84% (21/25), respectively. SWE images of the trapezius muscle for these patients were predominantly yellow. SWVmean values for severe pain MPS patients were greater than 4.00 m/s in all cases (100%, 7/7). SWE images of the trapezius muscle for these patients were predominantly red (Figures 5,6).
Correlation analysis
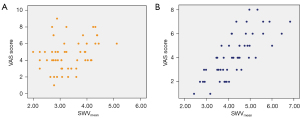
In the non-MPS patients, the correlation between SWVmean and VAS pain score was weak (r=0.329; 95% CI: 0.054–557; P=0.012), while it was strong in MPS patients (r=0.763; 95% CI: 0.614–0.852; P<0.001; Figure 7).
Diagnostic efficacy
The AUC of the upper trapezius SWVmean in MPS patients was 0.791 (95% CI: 0.703–0.863). The optimal SWVmean cut-off for diagnosing MPS patients was 3.43 m/s, and the sensitivity, specificity, and Youden index were 86.27% (44/51; 95% CI: 73.7–94.3%), 62.07% (36/58; 95% CI: 48.4–74.5%), and 0.48 (95% CI: 0.32–0.60), respectively (Figure 8A and Table 4).
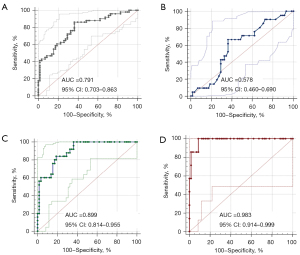
Table 4
| MPS | Optimal cut-off point (m/s) | AUC | Sensitivity (%) | Specificity (%) | Youden index |
|---|---|---|---|---|---|
| Total | ≥3.43 (>3.16 to >4.15) | 0.791 (0.703–0.863) | 86.27 (73.7–94.3) | 62.07 (48.4–74.5) | 0.48 (0.32–0.60) |
| Mild pain MPS | ≥3.43 (>2.83 to >4.04) | 0.578 (0.460–0.690) | 63.16 (38.4–83.7) | 62.07 (48.4–74.5) | 0.25 (0.11–0.42) |
| Moderate pain MPS | ≥4.00 (>3.31 to >4.39) | 0.899 (0.814–0.955) | 84.00 (63.9–95.5) | 81.03 (68.6–90.1) | 0.65 (0.48–0.74) |
| Severe pain MPS | ≥4.32 (>4.04 to >4.45) | 0.983 (0.914–0.999) | 100.00 (59.0–100.0) | 91.38 (81.0–97.1) | 0.91 (0.81–0.98) |
Data in parentheses are 95% confidence intervals. The optimal cut-off value was obtained at the point closest to the upper left of the receiver operating characteristic curve. SWVmean, mean shear-wave velocity; MPS, myofascial pain syndrome; AUC, area under the curve.
For the analysis of MPS patients grouped on the basis of pain severity, AUC values for SWVmean in patients with mild, moderate, and severe pain were 0.578 (95% CI: 0.460–0.690), 0.899 (95% CI: 0.814–0.955), and 0.983 (95% CI: 0.914–0.999), respectively (Figure 8B-8D). The optimal SWVmean cut-off point for diagnosing MPS in patients with mild pain was 3.43 m/s, and the sensitivity, specificity, and Youden index were 63.16% (95% CI: 38.4–83.7%), 62.07% (95% CI: 48.4–74.5%), and 0.25 (95% CI: 0.11–0.42), respectively. The optimal cut-off in patients with moderate pain was 4.00 m/s, and the sensitivity, specificity, and Youden index were 84.00% (95% CI: 63.9–95.5%), 81.03% (95% CI: 68.6–90.1%), and 0.65 (95% CI: 0.48–0.74). The optimal cut-off point in patients with severe pain was 4.32 m/s, and the sensitivity, specificity, and Youden index were 100.0% (95% CI: 59.0–100.0%), 91.38% (95% CI: 81.0–97.1%), and 0.91 (95% CI: 0.81–0.98), respectively (Table 4). The diagnostic efficacy of trapezius SWVmean for MPS was significantly higher in MPS patients with moderate and severe pain compared with patients with mild pain.
Discussion
Cervical MPS is characterized by sensory, motor, and autonomic symptoms caused by intramuscular MTrPs in the neck, and its main symptom is pain (6). Because of the absence of morphological changes in the muscles involved in MPS, imaging diagnostic criteria remain unavailable. Current diagnosis relies primarily on clinician experience, and inter-examiner reproducibility is low (12-14). Thus, objective and reliable imaging diagnostic indicators of MPS are needed (6,13-15).
Skeletal muscle fatigue is the most important underlying mechanism in MPS (8,34,35). According to The EFSUMB Guidelines and Recommendations for Musculoskeletal Ultrasound for the classification of endogenous skeletal muscle injury, skeletal muscle fatigue is considered a grade-0 injury, which reflects a lack of morphological change in the affected muscle and positive ultrasonographic findings (36). In this study, trapezius thickness, cross-sectional area, and RI did not differ between MPS and non-MPS patients. Previous studies have found that skeletal muscle fatigue injury in MPS is mainly manifested as delayed or diminished muscle relaxation that results in changes in elasticity mechanics (6,8,35). Ballyns et al. reported that muscle fatigue is associated with changes in skeletal muscle elasticity and increased stiffness (28). Similarly, we found significantly higher SWVmean values for the trapezius in MPS patients compared with non-MPS patients (a difference of 0.96 m/s, 95% CI: 0.65–1.28 m/s), which suggests that cervical MPS patients exhibit elasticity changes and increased stiffness in the trapezius muscle.
Pain, which is listed by the World Health Organization as “the fifth vital sign” besides respiration, heartbeat, pulse, and blood pressure, is the chief complaint of MPS patients. Therefore, objective and accurate evaluation of pain in MPS patients is crucial. However, the International Pain Society notes that pain is highly subjective and easily influenced by factors such as psychology and social environment (11). Moreover, most currently used pain assessment methods require a certain level of comprehension and communication abilities, making them unreliable for patients with poor communication abilities and comprehension (10,12). Additionally, psychological illnesses, such as depression, may accompany pain. MPS patients who also suffer from depression have lower pain thresholds than those without depression, which can affect objective assessment. Therefore, finding an objective and reliable index for evaluating MPS patients is the focus of the current study. Ertekin et al. found significantly higher muscle stiffness and pain levels in patients with trapezius MPS compared with a control group (37). Their results are consistent with ours. Our study further classified MPS patients according to the degree of pain, and showed that trapezius SWVmean did not differ between non-MPS and MPS patients with mild pain. However, it was significantly higher in MPS patients with moderate and severe pain compared with non-MPS patients (differences of 1.25 and 2.07 m/s, respectively). We also revealed a positive correlation between SWVmean and VAS pain score in MPS patients. Therefore, changes in trapezius stiffness may reflect MPS severity. When evaluating cervical MPS patients, examining changes in trapezius elasticity may prevent the over- or underestimation of pain severity, which is particularly important in patients with psychological or cognitive comorbidities.
At present, the diagnosis of MPS mainly relies on clinician experience. Diagnoses are often missed because of inadequate experience (13-15), especially in patients with cervical MPS and cervical spondylosis or other diseases. In patients with a chief complaint of neck pain, determining whether cervical MPS is present is essential. If so, subsequent decisions should focus on whether to treat this condition in conjunction with any other disorders that are concomitantly diagnosed, such as cervical spondylosis. Evaluations based on objective factors can prevent missed diagnoses. Numerous studies have found that changes in skeletal muscle elasticity occur because of pathology, suggesting that the evaluation of elasticity changes may be valuable for the diagnosis of MPS (16,19-26). In our study, trapezius SWVmean was significantly higher in MPS patients compared with non-MPS patients, and the diagnostic efficacy for cervical MPS (AUC value of 0.791) had an optimal cut-off of 3.43 m/s. Thus, MPS should be considered in patients with neck pain and SWVmean values greater than 3.43 m/s. However, elasticity measurements from the trapezius muscle in the neck of MPS patients obtained using real-time ultrasound shear-wave elastography carried high sensitivity but relatively low specificity. In addition, the diagnostic efficacy of SWVmean was significantly higher in patients with moderate (AUC =0.899) and severe pain (AUC =0.983) compared with patients affected by mild pain (AUC =0.578). Therefore, measuring trapezius SWVmean using shear-wave elastography may be useful for screening MPS, especially in patients with moderate to severe pain. Moreover, its use may prevent missed diagnoses.
An additional consideration is relevant in this context. Because there is little clinical need to use diagnostic tests for distinguishing between healthy people without neck pain and patients with MPS, the accuracy of diagnostic tests may be overestimated by selecting patients from these two categories in case-control studies. For this reason, we constructed our control group using non-MPS patients with clinical symptoms of neck pain that were equivalent to those manifested by MPS patients. This design choice allowed us to obtain accurate estimates of sensitivity and specificity.
Our study presents several limitations. First, we only examined young and middle-age patients with BMI between 18.5 and 23.9 kg/m2 because age and BMI affect muscle elasticity (16,19,36-38). In addition, we included only patients with right cervical MPS, and we only evaluated elasticity in the right trapezius muscle to avoid issues with neck muscle differences that may vary because of hand dominance and arm exercise (39). Our findings may not generalize to other patient populations.
Second, estimates of SWV, the elastic mechanical parameter of skeletal muscle, are affected by various factors. SWV measurements can vary depending on the machine used, tendon position, and the plane of imaging. In addition, sex, age, training and health status affect SWV (16,19,36). Therefore, studies using a larger sample size are needed to confirm our findings.
Furthermore, although shear-wave elastography in evaluating pathological muscle tension carries high sensitivity, its stability is insufficient (16,19). Selecting an appropriate body position to maintain skeletal muscle in the same state is crucial for accuracy (16,19). Moreover, the probe must be perpendicular to the interface, little pressure should be applied, and smooth patient breathing is also necessary to obtain satisfactory results (16). Therefore, sonographers must be well-trained and experienced to evaluate muscle elasticity in MPS patients.
Conclusions
Elasticity changes and increased stiffness in the trapezius muscle occur in patients with cervical MPS. Furthermore, the elasticity of the trapezius muscle of the neck of MPS patients assessed using real-time ultrasound shear-wave elastography demonstrated high sensitivity but relatively low specificity. The trapezius muscle elasticity parameter SWVmean may be valuable in the screening of cervical MPS, especially in patients with moderate and severe pain.
Acknowledgments
We thank Sarina Iwabuchi, PhD, from Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-797/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-797/coif). QX is a staff member at the Business Division of Hologic (Shanghai) Medical Supplies Co., Ltd. and assisted with the scientific research for data processing. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved (No. YXLL-2020-030) by the ethics review committee of the Third Hospital of Shanxi Medical University (Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital). Informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cerezo-Téllez E, Torres-Lacomba M, Mayoral-Del Moral O, Sánchez-Sánchez B, Dommerholt J, Gutiérrez-Ortega C. Prevalence of Myofascial Pain Syndrome in Chronic Non-Specific Neck Pain: A Population-Based Cross-Sectional Descriptive Study. Pain Med 2016;17:2369-77. [Crossref] [PubMed]
- Safiri S, Kolahi AA, Hoy D, Buchbinder R, Mansournia MA, Bettampadi D, Ashrafi-Asgarabad A, Almasi-Hashiani A, Smith E, Sepidarkish M, Cross M, Qorbani M, Moradi-Lakeh M, Woolf AD, March L, Collins G, Ferreira ML. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ 2020;368:m791. [Crossref] [PubMed]
- Gerwin RD. Diagnosis of myofascial pain syndrome. Phys Med Rehabil Clin N Am 2014;25:341-55. [Crossref] [PubMed]
- Liu L, Huang QM, Liu QG, Ye G, Bo CZ, Chen MJ, Li P. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96:944-55. [Crossref] [PubMed]
- Muñoz-Muñoz S, Muñoz-García MT, Alburquerque-Sendín F, Arroyo-Morales M, Fernández-de-las-Peñas C. Myofascial trigger points, pain, disability, and sleep quality in individuals with mechanical neck pain. J Manipulative Physiol Ther 2012;35:608-13. [Crossref] [PubMed]
- Simons DG, Travel JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual, Volume 1. Upper Half of Body. 2nd edition. Baltimore (MD): Williams & Wilkins/Wolters Kluwer Health Inc.; 1999
- Ribeiro DC, Belgrave A, Naden A, Fang H, Matthews P, Parshottam S. The prevalence of myofascial trigger points in neck and shoulder-related disorders: a systematic review of the literature. BMC Musculoskelet Disord 2018;19:252. [Crossref] [PubMed]
- Hagberg M, Kvarnström S. Muscular endurance and electromyographic fatigue in myofascial shoulder pain. Arch Phys Med Rehabil 1984;65:522-5. [PubMed]
- Money S. Pathophysiology of Trigger Points in Myofascial Pain Syndrome. J Pain Palliat Care Pharmacother 2017;31:158-9. [Crossref] [PubMed]
- Boonstra AM, Schiphorst Preuper HR, Reneman MF, Posthumus JB, Stewart RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008;31:165-9. [Crossref] [PubMed]
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976-82. [Crossref] [PubMed]
- Shafshak TS, Elnemr R. The Visual Analogue Scale Versus Numerical Rating Scale in Measuring Pain Severity and Predicting Disability in Low Back Pain. J Clin Rheumatol 2021;27:282-5. [Crossref] [PubMed]
- Hsieh CY, Hong CZ, Adams AH, Platt KJ, Danielson CD, Hoehler FK, Tobis JS. Interexaminer reliability of the palpation of trigger points in the trunk and lower limb muscles. Arch Phys Med Rehabil 2000;81:258-64. [Crossref] [PubMed]
- Rathbone ATL, Grosman-Rimon L, Kumbhare DA. Interrater Agreement of Manual Palpation for Identification of Myofascial Trigger Points: A Systematic Review and Meta-Analysis. Clin J Pain 2017;33:715-29. [Crossref] [PubMed]
- Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome--evidence from a review of the literature. Clin J Pain 2007;23:278-86. [Crossref] [PubMed]
- Săftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, Amy D, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med 2019;40:425-53. [Crossref] [PubMed]
- Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, Zoppo A. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid 2008;18:523-31. [Crossref] [PubMed]
- Schaefer FK, Heer I, Schaefer PJ, Mundhenke C, Osterholz S, Order BM, Hofheinz N, Hedderich J, Heller M, Jonat W, Schreer I. Breast ultrasound elastography--results of 193 breast lesions in a prospective study with histopathologic correlation. Eur J Radiol 2011;77:450-6. [Crossref] [PubMed]
- Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med 2013;34:238-53. [Crossref] [PubMed]
- Aubry S, Nueffer JP, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology 2015;274:821-9. [Crossref] [PubMed]
- Brandenburg JE, Eby SF, Song P, Kingsley-Berg S, Bamlet W, Sieck GC, An KN. Quantifying passive muscle stiffness in children with and without cerebral palsy using ultrasound shear wave elastography. Dev Med Child Neurol 2016;58:1288-94. [Crossref] [PubMed]
- Du LJ, He W, Cheng LG, Li S, Pan YS, Gao J. Ultrasound shear wave elastography in assessment of muscle stiffness in patients with Parkinson's disease: a primary observation. Clin Imaging 2016;40:1075-80. [Crossref] [PubMed]
- Eby S, Zhao H, Song P, Vareberg BJ, Kinnick R, Greenleaf JF, An KN, Chen S, Brown AW. Quantitative Evaluation of Passive Muscle Stiffness in Chronic Stroke. Am J Phys Med Rehabil 2016;95:899-910. [Crossref] [PubMed]
- Illomei G, Spinicci G, Locci E, Marrosu MG. Muscle elastography: a new imaging technique for multiple sclerosis spasticity measurement. Neurol Sci 2017;38:433-9. [Crossref] [PubMed]
- Lacourpaille L, Hug F, Guével A, Péréon Y, Magot A, Hogrel JY, Nordez A. Non-invasive assessment of muscle stiffness in patients with Duchenne muscular dystrophy. Muscle Nerve 2015;51:284-6. [Crossref] [PubMed]
- Lee SS, Spear S, Rymer WZ. Quantifying changes in material properties of stroke-impaired muscle. Clin Biomech (Bristol, Avon) 2015;30:269-75. [Crossref] [PubMed]
- Adigozali H, Shadmehr A, Ebrahimi E, Rezasoltani A, Naderi F. Reliability of assessment of upper trapezius morphology, its mechanical properties and blood flow in female patients with myofascial pain syndrome using ultrasonography. J Bodyw Mov Ther 2017;21:35-40. [Crossref] [PubMed]
- Ballyns JJ, Turo D, Otto P, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J Ultrasound Med 2012;31:1209-19. [Crossref] [PubMed]
- Chen Q, Wang HJ, Gay RE, Thompson JM, Manduca A, An KN, Ehman RE, Basford JR. Quantification of Myofascial Taut Bands. Arch Phys Med Rehabil 2016;97:67-73. [Crossref] [PubMed]
- Valera-Calero JA, Sánchez-Jorge S, Buffet-García J, Varol U, Gallego-Sendarrubias GM, Álvarez-González J. Is Shear-Wave Elastography a Clinical Severity Indicator of Myofascial Pain Syndrome? An Observational Study. J Clin Med 2021; [Crossref] [PubMed]
- Sikdar S, Ortiz R, Gebreab T, Gerber LH, Shah JP. A new application of ultrasound imaging to characterize tissue properties and blood flow in myofascial pain syndromes. 2010 IEEE International Ultrasonics Symposium; 11-14 October 2010; San Diego, CA, USA. IEEE; 2010:1380-3.
- Tavare AN, Alfuraih AM, Hensor EMA, Astrinakis E, Gupta H, Robinson P. Shear-Wave Elastography of Benign versus Malignant Musculoskeletal Soft-Tissue Masses: Comparison with Conventional US and MRI. Radiology 2019;290:410-7. [Crossref] [PubMed]
- Alfuraih AM, O'Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J Clin Ultrasound 2018;46:108-15. [Crossref] [PubMed]
- Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med 1989;151:157-60. [PubMed]
- Mannion AF, Dolan P. Relationship between myoelectric and mechanical manifestations of fatigue in the quadriceps femoris muscle group. Eur J Appl Physiol Occup Physiol 1996;74:411-9. [Crossref] [PubMed]
- Fodor D, Rodriguez-Garcia SC, Cantisani V, Hammer HB, Hartung W, Klauser A, et al. The EFSUMB Guidelines and Recommendations for Musculoskeletal Ultrasound - Part I: Extraarticular Pathologies. Ultraschall Med 2022;43:34-57. [Crossref] [PubMed]
- Ertekin E, Kasar ZS, Turkdogan FT. Is early diagnosis of myofascial pain syndrome possible with the detection of latent trigger points by shear wave elastography? Pol J Radiol 2021;86:e425-31. [Crossref] [PubMed]
- Kuo WH, Jian DW, Wang TG, Wang YC. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med Biol 2013;39:1356-61. [Crossref] [PubMed]
- Bedewi MA, Alhariqi BA, Aldossary NM, Gaballah AH, Sandougah KJ. Shear wave elastography of the scalene muscles in healthy adults: A preliminary study. Medicine (Baltimore) 2021;100:e26891. [Crossref] [PubMed]



