Intraventricular hemorrhage in term neonates with hypoxic-ischemic encephalopathy: a comparison study between neonates treated with and without hypothermia
Introduction
Hypoxic-ischemic encephalopathy (HIE) in neonates is a serious temporary or permanent dysfunction of the central nervous system resulting from perinatal brain asphyxia. Moderate or severe HIE affects 0.5–1 per 1,000 live births in the Western world and causes significant mortality and morbidity with over 25% of children experiencing long-term neurodevelopmental disability (1). The only neuroprotective treatment currently known to improve the outcome in HIE is therapeutic hypothermia. This therapy consists of whole body or selective head cooling to a basal ganglia temperature of about 32–34 degrees Celsius for 3 days to prevent reperfusion injury.
A meta-analysis of several major studies including the CoolCap study (2), the National Institute of Child Health and Human Development (NICHD) study (3), and the TOBY trial (4), has demonstrated a reduction in death and neurological impairment at 18 months following hypothermia (5). A Cochrane Collaboration review on the safety of this therapeutic approach has shown thrombocytopenia and hypotension to be its major side-effects (1). Nevertheless, some authors have also raised concerns that hypothermia might increase the risk of intraventricular hemorrhage (IVH) (6,7).
IVH and HIE were long believed to be pathologically interrelated. Yet, the incidence of IVH in term neonates with HIE is not well documented. In a study of factors affecting outcome in HIE in term infants in 1983, four cases of IVH were reported in 43 infants with HIE who had undergone computed tomographic scans (8). A recent study documented an IVH incidence of 9% in term asphyxiated newborns (9). Our study uses MRI and head ultrasound (HUS) to compare the incidence of IVH in term babies with HIE treated by therapeutic hypothermia versus those managed conventionally.
To retrospectively determine the overall prevalence of IVH in full-term neonates with HIE using HUS and MRI, and to determine whether there is an association between hypothermia and an increase in IVH.
Methods
A total of 61 term neonates from two institutions were diagnosed with HIE shortly after birth. Thirty infants from one institution were treated with whole body hypothermia and 31 neonates from the other institution received conventional care. All the neonates underwent HUS in their first 23 days of life. The 54 survivors also underwent MRI. The imaging studies were all reviewed for IVH. Presence of IVH on HUS was defined as increased echogenicity and size of the choroid plexus with or without ventricular dilatation and extension to the adjacent brain parenchyma. On MRI, IVH was defined according to hemorrhage limited to choroid plexus or extending into the lateral ventricles and cerebral parenchyma, using well known criteria (9).
From November 2008 to November 2010, 30 consecutive term newborns with HIE were treated with therapeutic hypothermia at the first institution (Group 1). These infants had to satisfy the entry criteria for neonatal hypothermia protocol of the institution. According to these standards, the administration of whole body hypothermia therapy is based on physiologic indications of hypoxia (criteria A) and neurologic signs (criteria B), both of which must be met. According to criteria A, if a cord or postnatal blood gas is available, there must be a pH ≤7.0 or a base deficit ≥16.0 mEq/L. If no blood gas is available, the pH is 7.01 to 7.15, or the base deficit is 10 to 15.9 mEq/L, there must be a documented acute perinatal event as well as an Apgar score ≤5 at 10 minutes or a continued need for ventilation initiated at birth and continued for at least 10 minutes. In keeping with criteria B, the child must exhibit signs of moderate to severe encephalopathy defined as seizures or presence of one or more signs in three of the following six categories: level of consciousness, spontaneous activity, posture, tone, primitive reflexes, and autonomic system. Babies in whom hypothermia could not be initiated within the first 6 hours of life, or infants with a severely abnormal aEEG tracing, a known chromosomal abnormality, a major congenital abnormality, a weight <1,800 g, a gestational age <36 weeks, evidence of severe injury, or multi-organ system failure were not eligible candidates for hypothermia. At the first institution, HUS was performed within the first 2 days of life by a pediatric radiologist with 14 years of experience using a Toshiba Aplio XG unit (Toshiba Medical Systems, Japan) with a standard protocol using a vector 9S4 MHz and linear-array transducer 11LW4 MHz. The MRI examinations were performed at the completion of the hypothermia treated on a MRI-3 T clinical System (Achieva X, Philips Healthcare) and were interpreted by a pediatric neuroradiologist with 15 years of experience. Each MRI examination included 3D T1-weighted gradient-echo, T2-weighted, gradient-echo, and diffusion-weighted imaging sequences.
The 31 consecutive babies who underwent conventional treatment for HIE were selected from December 2001 to April 2004 at the second institution (Group 2) (10). At that time, hypothermia was not yet a standard of care at that institution, and neonates of all Sarnat stages were included in this group. Neonates with severe congenital abnormalities, metabolic abnormalities, or infectious disease were excluded from the study. At the second institution, HUS was performed using Acuson sequoia (Siemens, Mountain view CA, USA) or ATL 5000 (Philips, Bothel, WA, USA) machines with vector, curved, and linear array transducers ranging between 8–15 MHz by three pediatric radiology fellows, each with 3 years of experience. The US examinations were reviewed by a pediatric radiologist with 25 years of experience. MRI examinations were carried out on a 1.5 Tesla GE Signa (General Electric, Milwaukee, WI, USA) machine and were interpreted by a pediatric neuroradiologist with 18 years of experience. Spin echo sequences and diffusion-weighted images were performed in all patients. At both institutions, the HUS reader was blinded the MRI findings and the MRI reader was blinded to the HUS findings.
Results
Amongst the 30 babies who received whole body hypothermia, there were 18 males and 12 females, the mean birth weight was 3.5 kg (2.5 to 5.2 kg), and the HUS study was performed within 14.8 to 41 hours of life (Table 1). The group of 31 infants treated conventionally was comprised of 12 boys and 19 girls, the infants had an average birth weight of 3.3 kg (2.3 to 4.2 kg), and they underwent HUS 1 to 23 days after birth, with only five children being older than 1 week at the time of the imaging studies. There was no statistically significant difference in the gender distribution between the two groups (P=0.096, chi-square test).

Full table
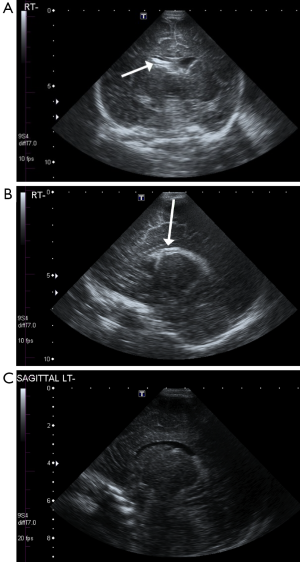
Four of the 61 infants (6.6%) were diagnosed with IVH on HUS. In the group of neonates treated with hypothermia, there were three cases (10%) of IVH. The first child with IVH had an obvious, unilateral right intraventricular bleed seen on HUS (Figure 1). The second case showed bilateral hemorrhage which was not evident on HUS due to symmetry, but which was confirmed by MRI. In the third infant, HUS revealed bilateral enlarged choroid plexus. However, IVH could not be confirmed with MRI, as the baby did not survive. The former two children were classified as having HIE of Sarnat stage II and the latter infant as Sarnat stage III.
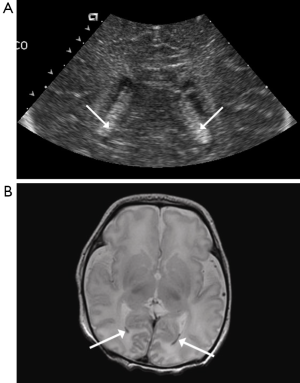
In the group not subjected to hypothermia, IVH occurred in one infant (3.2%). The hemorrhage was bilateral and was noted both on US and MRI (Figure 2). The difference in rate of IVH between the two groups was not statistically significant (P=0.354, Fisher’s exact test).
Discussion
IVH is well documented in premature infants. It is much less common in term infants, although rates of 3% have been reported in healthy term babies studied with HUS (11). Unlike in pre-term children in whom IVH originates in the germinal matrix, neuropathological studies demonstrate that the site of bleeding in term neonates is usually the choroid plexus (12). Germinal matrix hemorrhage occurs only in a minority of term neonates with IVH, and usually involves the caudothalamic groove (13,14). The proposed reason for this difference is that the high cellularity and vascularity of the germinal matrix progressively disappear from 24 to 32 weeks of gestation as the brain matures (15-17). Moreover, there are differences in regional cerebral blood-flow: as the baby matures, the majority of the blood flow is directed to the cortex and white matter in contrast to the basal ganglia and periventricular regions that are most highly perfused in premature infants (16). This hemodynamic change might also explain the lower incidence of IVH in term infants (14).
The pathogenesis of IVH is multifactorial and no clear mechanism is known. An altered autoregulation, hemodynamic instability with fluctuating blood flow and arterial or venous pressure, early filling of the deep venous system, immaturity of the capillary bed, poorly supported capillaries, and coagulation disturbances may all contribute to vessel injury (13,17-20). Moreover, sinovenous thrombosis has been found to be implicated in almost a third of thalamic hemorrhage in term neonates with IVH (11).
In our study, IVH occurred at a higher rate in children who underwent hypothermia (10%) than in those treated conventionally (3%). This difference is not statistically significant, which may be due to the small size of our cohort. Moreover, this difference might not necessarily reflect a higher risk of IVH following hypothermia, but rather the selection of clinically more severe cases for the hypothermia treatment. Indeed, the inclusion criteria stipulate that only neonates presenting with Sarnat stage II or III (21) be considered eligible candidates for the procedure. Higher Sarnat stages indicate poorer prognosis and outcomes as well as an increased risk of complications: Term neonates with mild encephalopathy are often normal at follow-up, whereas those at a moderate stage have sequalae 20% to 35% of the time, and infants with severe brain damage almost always present some long-term complications and have a 75% risk of death in the neonatal period (22). In addition, our study may be limited by an imperfect reference standard, given the five outliers in group 2 who had a HUS after their 1st week of age.
On the other hand, hypothermia has been associated with significantly increased thrombocytopenia, which is an independent risk factor for IVH (1,17,19). It has also been found to increase the need for inotrope support to treat hypotension, which may contribute to hemodynamic instability, a factor that might predispose to IVH.
Conclusions
IVH appears uncommon in term infants with HIE, occurring at a rate of 7% in our series. However, it was more prevalent in the group treated with hypothermia (10% vs. 3%). The higher prevalence of IVH in neonates treated with hypothermia may not necessarily be a consequence of the therapy. It might reflect the selection protocol of more clinically severe cases of HIE in the hypothermia group. It remains to be determined whether a difference in IVH incidence between the hypothermia group and the control group persists. An alternative may be to perform HUS before and during hypothermia to monitor for the development of IVH in term neonates with HIE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional ethics board of Montreal Children’s Hospital for retrospective review in 2010 and by Institutional Research Ethics Board of Hospital for Sick Children for Neonatal encephalopathy study performed from 2001−2004.
References
- Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2003.CD003311. [PubMed]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663-70. [Crossref] [PubMed]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574-84. [Crossref] [PubMed]
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P, TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349-58. [Crossref] [PubMed]
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363. [Crossref] [PubMed]
- Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, Thoresen M. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005;116:1001-6. [Crossref] [PubMed]
- Fatemi A, Wilson MA, Johnston MV. Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol 2009;36:835-58. vii. [Crossref] [PubMed]
- Finer NN, Robertson CM, Peters KL, Coward JH. Factors affecting outcome in hypoxic-ischemic encephalopathy in term infants. Am J Dis Child 1983;137:21-5. [PubMed]
- Al Yazidi G, Boudes E, Tan X, Saint-Martin C, Shevell M, Wintermark P. Intraventricular hemorrhage in asphyxiated newborns treated with hypothermia: a look into incidence, timing and risk factors. BMC Pediatr 2015;15:106. [Crossref] [PubMed]
- Epelman M, Daneman A, Kellenberger CJ, Aziz A, Konen O, Moineddin R, Whyte H, Blaser S. Neonatal encephalopathy: a prospective comparison of head US and MRI. Pediatr Radiol 2010;40:1640-50. [Crossref] [PubMed]
- Wu YW, Hamrick SE, Miller SP, Haward MF, Lai MC, Callen PW, Barkovich AJ, Ferriero DM. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol 2003;54:123-6. [Crossref] [PubMed]
- Haas RH. Vascular Disease. In: David R. editor. Child and Adolescent Neurology. 2 Edition. Massachusetts: Wiley, 2005:279.
- Bergman I, Bauer RE, Barmada MA, Latchaw RE, Taylor HG, David R, Painter MJ. Intracerebral hemorrhage in the full-term neonatal infant. Pediatrics 1985;75:488-96. [PubMed]
- Fink S. Intraventricular hemorrhage in the term infant. Neonatal Netw 2000;19:13-8. [Crossref] [PubMed]
- Donat JF, Okazaki H, Kleinberg F, Reagan TJ. Intraventricular hemorrhages in full-term and premature infants. Mayo Clin Proc 1978;53:437-41. [PubMed]
- Lacey DJ, Terplan K. Intraventricular hemorrhage in full-term neonates. Dev Med Child Neurol 1982;24:332-7. [Crossref] [PubMed]
- Adcock LM. Clinical manifestations and diagnosis of intraventricular hemorrhage in the newborn. UpToDate 2011. Available online: http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-intraventricular-hemorrhage-in-the-newborn
- Perlman JM, Volpe JJ. Prevention of neonatal intraventricular hemorrhage. Clin Neuropharmacol 1987;10:126-42. [Crossref] [PubMed]
- Scher MS, Wright FS, Lockman LA, Thompson TR. Intraventricular hemorrhage in the full-term neonate. Arch Neurol 1982;39:769-72. [Crossref] [PubMed]
- Hill A, Volpe JJ. Seizures, hypoxic-ischemic brain injury, and intraventricular hemorrhage in the newborn. Ann Neurol 1981;10:109-21. [Crossref] [PubMed]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696-705. [Crossref] [PubMed]
- Wu Y. Clinical features, diagnosis, and treatment of neonatal encephalopathy. UpToDate 2011. Available online: http://www.uptodate.com/contents/clinical-features-diagnosis-and-treatment-of-neonatal-encephalopathy


