
Multiple myeloma with diffuse uptake on 18F-PSMA-1007 positron emission tomography/computed tomography: a case description and literature review
Introduction
18F-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in the imaging of prostate cancer is now well-established (1). Despite excellent sensitivities, it is noteworthy that PSMA expression is not specific to the prostate; increased 18F-PSMA uptake can be found in some non-prostatic malignancies (2). Herein, we present the case of a 76-year-old male patient who underwent 18F-PSMA-1007 PET/CT for bone pain after radical prostatectomy. PET images revealed increased 18F-PSMA uptake of diffuse bone lesions and CT images showed multiple osteolytic destructions. Bone biopsy confirmed a diagnosis of multiple myeloma (MM). We also summarize the histopathology, clinical characteristics, and imaging manifestations of MM, especially regarding PSMA PET/CT imaging.
Case presentation
A 76-year-old male patient underwent prostatectomy for prostate cancer and was followed for more than 9 months. During the whole follow-up process, his prostate-specific antigen (PSA) level was stable with a most recent PSA reading of 0.006 ng/mL in our hospital. Clinically, there was no evidence of biochemical recurrence after surgery. The patient experienced pain in the anterior chest wall and back for more than 1 month, and 18F-PSMA-1007 PET/CT imaging was performed to further investigate the possibility of recurrence and bone metastases.
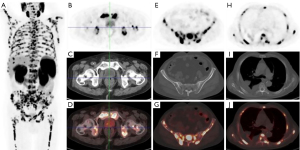
18F-PSMA-1007 PET/CT imaging showed no abnormal PSMA uptake in the prostatectomy area, however, extensive PSMA-avid osteolytic lesions were found in multiple bones (including the skull, bilateral humerus, clavicle, scapula, sternum, ribs, multiple vertebral bodies of the spine, pelvis, bilateral humerus, and middle/upper femur). The maximum intensity projection (MIP) images of PET/CT showed extensive PSMA-avid osseous lesions in the skeleton, including the spine, bilateral humerus, and middle/upper femur (Figure 1A). Normal uptake was found in the prostatectomy region (Figure 1B-1D). In the pelvic region, multiple osteolytic lesions of the sacrum and bilateral iliac bones with intense PSMA uptake were found, and the maximum standardized uptake value (SUVmax) of the sacral lesion was about 31.0 (Figure 1E-1G). In the thoracic region, PET/CT images also showed many PSMA-avid osteolytic lesions in multiple vertebral bodies of the spine and bilateral ribs (Figure 1H-1J).
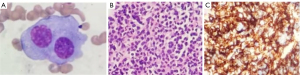
For further evaluation, histological specimens of the iliac bone marrow were obtained. The bone marrow aspiration cytology showed hyperplasia with visible and abnormal plasma cells (Figure 2A). The biopsy pathology showed abnormal cell proliferation of plasma cells with hypertrophy, abundant cytoplasm, round or irregular nucleoli, and reticulin fibrosis of Grade MF-3 (Figure 2B). The immunohistochemical (IHC) staining showed that CD138 (+), CD38 (+), kappa (+), lambda (−), MUM1 (+), CD56 (−), CyclinD1 (+), CD19 (−), cytokeratin (CK) cells cytoplasmic (+), PSA (−), P504S (−), positive rate of Ki67 <5% (Figure 2C). The final histological diagnosis was plasma cell myeloma (PCM). According to the Chinese guidelines for the diagnosis and treatment of MM (revised in 2020) (3), the final clinical diagnosis was IgG and kappa light chain-type MM. At the time of writing, our patient had received 6 courses of the BRD regimen (bortezomib + lenalidomide + dexamethasone) and was in full remission.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
MM is a hematological malignant neoplasm defined by the abnormal proliferation of clonal plasma cells, and it is the second most common tumor of the blood system after lymphoma. Males are more likely to have MM than females and the median age at the time of discovery is around 65 years old. Some of the most common clinical signs are osteolytic bone destruction, anemia, renal dysfunction, recurring infections, and hypercalcemia, and the disease progresses rapidly in a short period of time. MM presents as diffuse or focal bone infiltration, in which the destruction is usually confined to the bone marrow and extramedullary infiltrates are rare. Overall, extramedullary lesions occur in approximately 10–16% of all MM cases (4). The common diagnostic methods of MM are laboratory tests, urine testing, bone marrow biopsy, and imaging evaluations including CT, magnetic resonance imaging (MRI), whole-body scintigraphy, and PET (5). 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG) PET/CT provides both anatomical and metabolic information for the diagnosis, staging, treatment response, and prognostic assessment of MM (6).
PSMA, also known as glutamate carboxypeptidase II, is a transmembrane glycoprotein normally expressed in healthy human tissues and normal prostate epithelium, and it is strongly up-regulated in prostate tumor cells and related metastatic lesions (7). Its high sensitivity and high tumor background ratio make PSMA an excellent PET imaging agent for prostate cancer (8), and of great value in the accurate staging, metastasis evaluation, and treatment response assessment, especially in patients with biochemical recurrence (9). On the other hand, a variety of normal tissues express PSMA in salivary glands, lacrimal glands, the liver, spleen, pancreas, small intestine, bladder, and renal cortex. It is reported that PSMA can be highly expressed in other non-prostatic malignant tumors (such as glioblastoma and renal cell carcinoma) and some benign lesions (such as schwannoma and adrenal adenoma) (10-12).
MM is predominantly characterized by multifocal osteolysis on CT images. Tumor neovascularization occurs earlier than osteolytic changes by CT, a comprehensive result of osteoclast activation, osteoblast inhibition, and imbalance in the bone remodeling process (13), which results in bone defection. Besides, it is not possible to visualize osteolytic osseous destruction in the early period, in the case of preserved trabecular bone structure. Compared to CT, PET scan, using PSMA as the tracer, is more effective in evaluating bone destruction. Several experts (10,12,14-18) have reported cases of high PSMA uptake in MM and considered PSMA-avid mechanism as the excessive expression of PSMA in tumor neoangiogenesis. However, many previous studies utilized 68Ga-PSMA imaging (10,12,14-16) and the lesions in some cases were discrete (10,12,14-17). Only one of them was diffuse bone marrow involvement of MM on 18F-PSMA-1007 PET/CT without any pathological evidence of prostate cancer (18). Physically, the end-point positron energy of 18F-labeled PSMA ligands is much lower than that of 68Ga (0.65 vs. 1.90 MeV), which reduces the positron range in tissue and may improve spatial resolution (19). Whole-body PSMA tumor volume (wbPSMA-TV) and whole-body total lesions PSMA uptake (wbTL-PSMA) have been shown to predict progression-free survival, and wbPSMA-TV had the greatest accuracy among these parameters (20). In our MM case, on 18F-PSMA-1007 PET/CT, extensively intense uptakes were found in the axial skeleton including the skull, bilateral humerus, clavicle, scapula, sternum, ribs, multiple vertebral bodies of the spine, pelvis, bilateral humerus, and middle/upper femur. Non-prostatic pathological uptake is a potential pitfall of PSMA PET/CT imaging. Especially in this case, diffuse PSMA-avid bone lesions were mimicking the recurrence of prostate cancer, however, no evidence of biochemical recurrence was found, and the histological diagnosis was PCM. Overall, this case of MM found in a patient with a history of prostate cancer and with extensive bone uptakes on 18F-PSMA-1007 PET is extremely rare and a similar case has not been reported before. Therefore, more studies are needed to evaluate whether this diffuse uptake could be considered a suggestive feature of myeloma on 18F-PSMA-1007.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1310/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farolfi A, Calderoni L, Mattana F, Mei R, Telo S, Fanti S, Castellucci P. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J Nucl Med 2021;62:596-604. [Crossref] [PubMed]
- Van de Wiele C, Sathekge M, de Spiegeleer B, de Jonghe PJ, Beels L, Maes A. PSMA-Targeting Positron Emission Agents for Imaging Solid Tumors Other Than Non-Prostate Carcinoma: A Systematic Review. Int J Mol Sci 2019; [Crossref] [PubMed]
- Chinese Hematology Association. Chinese Myeloma Committee-Chinese Hematology Association. The guidelines for the diagnosis and management of multiple myeloma in China(2020 revision). Zhonghua Nei Ke Za Zhi 2020;59:341-6.
- Ferraro R, Agarwal A, Martin-Macintosh EL, Peller PJ, Subramaniam RM. MR imaging and PET/CT in diagnosis and management of multiple myeloma. Radiographics 2015;35:438-54. [Crossref] [PubMed]
- Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, Tuazon S, Gopal AK, Libby EN. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022;327:464-77. [Crossref] [PubMed]
- Zanoni L, Mattana F, Calabrò D, Paccagnella A, Broccoli A, Nanni C, Fanti S. Overview and recent advances in PET/CT imaging in lymphoma and multiple myeloma. Eur J Radiol 2021;141:109793. [Crossref] [PubMed]
- Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395:1208-16. [Crossref] [PubMed]
- Gupta P, Murthy V, Agarwal A, Maitre M, Mhatre N, Rangarajan V. 68Ga-prostate-specific membrane antigen PETCT-based response to androgen deprivation therapy in patients with prostate cancer. Nucl Med Commun 2019;40:1283-8. [Crossref] [PubMed]
- Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019;5:856-63. [Crossref] [PubMed]
- Rauscher I, Maurer T, Steiger K, Schwaiger M, Eiber M. Image of the Month: Multifocal 68Ga Prostate-Specific Membrane Antigen Ligand Uptake in the Skeleton in a Man With Both Prostate Cancer and Multiple Myeloma. Clin Nucl Med 2017;42:547-8. [Crossref] [PubMed]
- Strele-Trieb P, Dunzinger A, Sonnberger M, Wolfsgruber J, Pichler R. Uptake of 68Ga-Prostate-Specific Membrane Antigen PET in Adrenal Gland: A Potential Pitfall. Clin Nucl Med 2018;43:50-1. [Crossref] [PubMed]
- Alabed YZ. Multiple Solitary Plasmacytomas With Multifocal Bone Involvement Diagnosed With 68Ga-Prostate-Specific Membrane Antigen PET/CT. Clin Nucl Med 2020;45:e51-2. [Crossref] [PubMed]
- Gau YC, Yeh TJ, Hsu CM, Hsiao SY, Hsiao HH. Pathogenesis and Treatment of Myeloma-Related Bone Disease. Int J Mol Sci 2022; [Crossref] [PubMed]
- Sasikumar A, Joy A, Pillai MR, Nanabala R, Thomas B. 68Ga-PSMA PET/CT Imaging in Multiple Myeloma. Clin Nucl Med 2017;42:e126-7. [Crossref] [PubMed]
- Merrild EH, Baerentzen S, Bouchelouche K, Buus S. Vertebral Myeloma Mimicking Prostatic Carcinoma Metastasis in 68Ga-PSMA PET/CT. Clin Nucl Med 2017;42:790-2. [Crossref] [PubMed]
- Pan Q, Luo Y, Ma Y, Li F. The Change of 68Ga-Prostate-Specific Membrane Antigen Uptake in Myeloma After Chemotherapy in a Patient With Multiple Myeloma and Concurrent Prostate Cancer. Clin Nucl Med 2020;45:1013-5. [Crossref] [PubMed]
- Mittlmeier LM, Ledderose ST, Schott M, Brendel M, Beyer L, Theurich S, Mayr D, Walz C, Kunz WG, Ricke J, Bartenstein P, Ilhan H, Staehler M, Unterrainer M. Immature Plasma Cell Myeloma Mimics Metastatic Renal Cell Carcinoma on (18)F-PSMA-1007 PET/CT Due to Endothelial PSMA-Expression. Diagnostics (Basel) 2021; [Crossref] [PubMed]
- Michalski K, Jilg CA, Engelhardt M, Meyer PT, Ruf J. Diffuse Bone Marrow Involvement of Multiple Myeloma on [ 18 F]PSMA-1007 PET/CT: Is There a Theranostic Potential? Clin Nucl Med 2022;47:968-9. [Crossref] [PubMed]
- Hoberück S, Löck S, Borkowetz A, Sommer U, Winzer R, Zöphel K, Fedders D, Michler E, Kotzerke J, Kopka K, Hölscher T, Braune A. Intraindividual comparison of (68) Ga-Ga-PSMA-11 and (18)F-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res 2021;11:109. [Crossref] [PubMed]
- Zou Q, Jiao J, Zou MH, Li MZ, Yang T, Xu L, Zhang Y. Semi-automatic evaluation of baseline whole-body tumor burden as an imaging biomarker of (68)Ga-PSMA-11 PET/CT in newly diagnosed prostate cancer. Abdom Radiol (NY) 2020;45:4202-13. [Crossref] [PubMed]


