
Assessment of late on-set fetal growth restriction using SMI (superb microvascular imaging) Doppler
Introduction
Late on-set fetal growth restriction (FGR), which is diagnosed beyond 32 weeks, is caused by a mild or moderate placental insufficiency, with fewer and less specific histological findings. This explains why the pulsatile index (PI) of the umbilical artery (UA) is increased less frequently, thus its utility as a predictor for adverse perinatal outcome in these types of cases is quite limited (1). Even though placental insufficiency in late on-set FGR is less severe than in early on-set FGR, it still associates adverse perinatal outcomes and neurodevelopmental impairment (2). Despite this kind of FGR being the most common (70–80% of all FGR cases), the mechanism behind it still remains unknown, which hinders its identification, especially in at-term fetuses (3). Some authors have suggested the existence of a preclinical stage during which the fetus is exposed to a reduction of nutrients and oxygen support, which causes systemic adaptive responses that finally manifest in a growth reduction. Conventional Doppler might not reflect this disbalance between fetal needs and maternal support before the appearance of hypoxemia, thus the diagnosis is actually made once the adaptive fetal chain of response has been established (3,4). Moreover, some studies have found insufficiency findings in the placentas of small for gestational age (SGA) fetus with normal Doppler, and a higher risk of complications (5-7). This make us wonder whether within the SGA fetus group are undiagnosed FGR fetus which cannot be identified by current techniques (3,6).
Given the limitations of current diagnosis guidelines for late on-set FGR, some authors have focused their research in finding a direct way to assess placental function, with techniques such as 3-D Color Doppler or 3-D Power Doppler angiography (8-10). However, their usefulness has been questioned due to their poor reproducibility in the third trimester (11).
Superb microvascular imaging (SMI) Doppler is a rather recent technology based on the use of an algorithm that erases noise from breathing movements and surrounding tissues, allowing for the capture of low-velocity flow vessels, which has permitted authors to apply it to the depiction of placental findings (12-14). Sainz et al. were the first to apply this technique to normal pregnancies in a longitudinal study, establishing reference values throughout the pregnancy (15). It has been found to be more precise than conventional Doppler in the identification of the vessels of the villous tree (16), with an excellent intra- and interobserver reproducibility (17).
Hence, the aim of our study was to evaluate the diagnostic capability of SMI Doppler for the detection of placental insufficiency findings. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-807/rc).
Methods
A prospective observational study was carried out between December 2019 and May 2022 at tertiary care center, including 51 pregnant patients who had been diagnosed with SGA or FGR after the 32nd week. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received the approval of the Andalucia’s Board of Biomedicine Ethics Committee (No. 1001-N-18). All patients who agreed to participate gave their written informed consent.
Patients were consecutively recruited at the time of the diagnosis and were invited to participate in the study if they met the inclusion criteria. The inclusion criteria were the diagnosis after the 32nd week of late on-set SGA, defined as an estimated fetal weight (EFW) between the 3rd and 10th percentile, with a normal Doppler evaluation; or late on-set FGR, defined as an EFW below the 3rd percentile, or between the 3rd and 10th percentile with a pathologic Doppler. The exclusion criteria were: multiple pregnancies, previous history of systemic or obstetric diseases; a high-risk combined first-trimester screening; or the presence of pathologic findings in the 20th week morphologic scan.
Epidemiological variables collected were: maternal age, parity, previous cesarean section, smoking, body mass index (BMI) at the time of the evaluation, and weight gain during the pregnancy. We also collected variables related to obstetrics and neonatal outcomes, such as: development of preeclampsia, gestational age at the time of labor, on-set of labor, type of delivery, suspected intrapartum fetal distress, newborn weight, Apgar score below 7 at 1 and 5 minutes, umbilical cord pH, neonatal intubation, admission to neonatal intensive care unit (NICU), neonatal respiratory distress, neonatal hypoglycemia, newborn morbidity and newborn death.
Ultrasonographic assessment
Ultrasonography evaluation was performed by two expert examiners with more than 15 years of experience in fetal ultrasound using a Canon Aplio 500 ultrasound machine (Toshiba Medical Systems Corp., Tokyo, Japan) with a PUT-675 MV-3 probe. First, a conventional ultrasonography assessment was performed, using Color Doppler and Spectral Doppler, collecting the following parameters: EFW obtained from the combination of biometric measures (biparietal diameter head circumference, abdominal circumference, and femur length) (18), EFW percentile, PI and peak systolic velocity (PV) of the UA, middle cerebral artery (MCA), uterine arteries (UtAs), and ductus venosus (DV), as well as the cerebroplacental ratio (CPR). Afterwards, we performed the ultrasonographic evaluation of the placenta using SMI Doppler, which was applied to its central part. To this end, we followed the technique described by Sainz et al. (15). Quantitative parameters collected were the PI and PV of the basal plate, chorionic plate, and primary, secondary and tertiary villi (Figure 1). Qualitative parameters collected were the number of secondary and tertiary villi, classified as abundant or sparse depending on whether they occupied more than 50% of the Doppler gate.
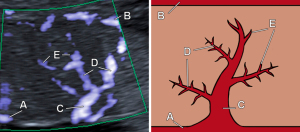
Histologic evaluation
After delivery, all placentas were submitted for histopathologic assessment, which was performed by one pathologist who was blinded to the ultrasonographic evaluation results (19). For the purpose of this study, a classification system was established based on the parameters that have proven to be more associated with FGR (20), such as infarction, the increase of syncytial knots, fibrinoid vascular necrosis, avascular distal villi, thrombosis, perivillous fibrin deposits, villitis and retroplacental hemorrhage. Hence, patients were classified into two groups, attending to the histologic findings of the placental: Group 1 (Normal group), with none of the listed items; and Group 2 (FGR group), with placental insufficiency, including those with the mentioned items.
Statistical analysis
The statistical analysis was carried out using the IBM SPSS Statistics software version 26 (IBM, Armonk, NY, USA). Mean and standard deviation were used to describe quantitative variables, while in the case of qualitative variables percentages were used. Normality of the data was evaluated with the Shapiro-Wilk test. First, a bivariate was carried out, using Student’s t-test for independent samples or Mann-Whitney U-test for quantitative variables, while the Chi-square test was used for qualitative variables. Statistical significance was set at 0.05. To detect at least a 55% difference between the PI and PV between normal and FGR placentas, with a 5% alpha error and an 80% power, we needed 20 patients per study group.
Results
The recruitment process of the study is summarized in Figure 2: out of 100 eligible patients, 22 were excluded, and 27 were lost to follow-up. Thus, a total of 51 patients were included in the study, sorted into two groups after the histologic examination: 21 belonging to Group 1 (Normal group), and 30 to Group 2 (FGR group). Mean age of patients was 28.1 years in the Normal group, and 29.1 years in the FGR group, without reaching statistical significance. Most patients were nulliparous (66.7% Normal group; 63.3% FGR group; P=0.81), with a 9.5% and 6.7% of patients having a previous cesarean section in groups 1 and 2, respectively. No statistically significant differences were found between groups (Table 1).
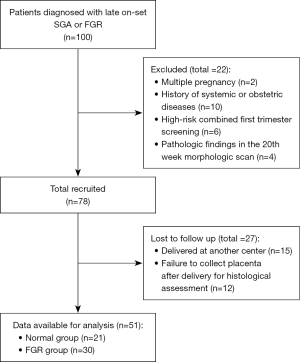
Table 1
| Variables | Group 1 (n=21) | Group 2 (n=30) | P |
|---|---|---|---|
| Maternal age (years) | 28.1±6.8 | 29.1±7.1 | 0.29 |
| Nulliparous | 14 (66.7) | 19 (63.3) | 0.81 |
| Previous cesarean section | 2 (9.5) | 2 (6.7) | >0.99 |
| Smoking | 6 (28.6) | 4 (13.3) | 0.18 |
| BMI (kg/m2) | 26.8 (7.1) | 24.8 (5.2) | 0.75 |
| Weight gain (kg) | 10.5±4.8 | 10.1±3.5 | 0.37 |
Parametric numeric variables are expressed as mean ± SD and non-parametric numeric variables are expressed as median (interquartile range), while qualitative variables are expressed as frequencies and percentages. Group 1: Normal group; Group 2: FGR group. BMI, body mass index; SD, standard deviation; FGR, fetal growth restriction.
Regarding obstetrics and neonatal outcomes (Table 2), there was a lower rate of eutocic deliveries in the FGR group (61.9% vs. 50%; P=0.43) and a higher rate of suspected intrapartum fetal distress (5.9% vs. 24.1%; P=0.12). There were also higher rates of Apgar scores below 7, as well as a higher neonatal hypoglycemia rate, in the FGR group, as displayed in Table 2. However, none of these observations reached statistical significance.
Table 2
| Variables | Group 1 (n=21) | Group 2 (n=30) | P |
|---|---|---|---|
| Preeclampsia | 2 (9.5) | 4 (13.3) | >0.99 |
| Gestational age at delivery (weeks) | 38 (2.0) | 38 (2.0) | >0.99 |
| On-set of labor | |||
| Spontaneous | 4 (19) | 6 (20.0) | 0.17 |
| Induced | 13 (61.9) | 23 (76.7) | |
| Elective cesarean | 4 (19) | 1 (3.3) | |
| Type of delivery | |||
| Eutocic delivery | 13 (61.9) | 15 (50.0) | 0.43 |
| Operative vaginal delivery | 3 (14.3) | 9 (30.0) | |
| Cesarean | 5 (23.8) | 6 (20.0) | |
| Suspected intrapartum fetal distress | 1 (5.9) | 7 (24.1) | 0.12 |
| Newborn weight (g) | 2,496±333 | 2,399±283 | 0.13 |
| Apgar score <7 at 1 minute | 0 (0) | 3 (10.0) | 0.26 |
| Apgar score <7 at 5 minutes | 0 (0) | 1 (3.3) | >0.99 |
| Umbilical cord pH | 7.31 (0.1) | 7.29 (0.1) | 0.21 |
| Neonatal intubation | 0 (0) | 1 (3.3) | >0.99 |
| Admission to NICU | 6 (28.6) | 10 (33.3) | 0.72 |
| Neonatal respiratory distress | 2 (9.5) | 3 (10.0) | >0.99 |
| Neonatal hypoglycemia | 1 (4.8) | 4 (13.3) | 0.39 |
| Newborn morbidity | 2 (9.5) | 3 (10.0) | >0.99 |
| Newborn death | 0 (0) | 0 (0) | – |
Parametric numeric variables are expressed as mean ± SD and non-parametric numeric variables are expressed as median (interquartile range), while qualitative variables are expressed as frequencies and percentages. Group 1: Normal group; Group 2: FGR group. NICU, neonatal intensive care unit; SD, standard deviation; FGR, fetal growth restriction.
The results of conventional Doppler assessment are displayed in Table 3. As expected, the FGR group had a lower CPR value than the Normal group (1.65 vs. 1.42; P=0.04). The rest of the parameters showed no statistically significant differences.
Table 3
| Conventional Doppler | Group 1 (n=21) | Group 2 (n=30) | P |
|---|---|---|---|
| EFW (g) | 2,511 (463.0) | 2,384 (300.0) | 0.55 |
| EFW percentile | 5 (6.0) | 3 (2.0) | 0.06 |
| PI UA | 0.93 (0.34) | 1.03 (0.23) | 0.07 |
| PV UA (cm/s) | 39.4±10.3 | 38.4±8.1 | 0.35 |
| PI MCA | 1.43±0.28 | 1.42±0.38 | 0.44 |
| PV MCA (cm/s) | 48.9±12.22 | 48.4±12.85 | 0.44 |
| CPR | 1.65±0.46 | 1.42±0.47 | 0.04 |
| PI DV | 0.35±0.17 | 0.42±0.14 | 0.06 |
| PV DV (cm/s) | 41.6±12.5 | 42.2±16.02 | 0.44 |
| Mean PI UtA | 0.76 (0.54) | 0.82 (0.4) | 0.53 |
| Mean PV UtA (cm/s) | 94.3 (57.7) | 76.6 (46.1) | 0.25 |
| Pathologic Doppler | 6 (30.0) | 14 (48.3) | 0.20 |
Parametric numeric variables are expressed as mean ± SD and non-parametric numeric variables are expressed as median (interquartile range), while qualitative variables are expressed as frequencies and percentages. Group 1: Normal group; Group 2: FGR group. EFW, estimated fetal weight; PI, pulsatile index; UA, umbilical artery; PV, peak systolic velocity; MCA, middle cerebral artery; CPR, cerebroplacental ratio; DV, ductus venous; UtA, uterine artery; SD, standard deviation; FGR, fetal growth restriction.
Results of SMI Doppler parameters are shown in Table 4. We found a lower PV of the chorionic plate in the FGR group (11 vs. 9; P=0.02). We observed that the PV of the other vessels were consistently lower in the FGR group that in the normal group, although without reaching statistical significance.
Table 4
| Variables | Group 1 (n=21) | Group 2 (n=30) | P |
|---|---|---|---|
| Gestational age (weeks) | 37 (2.0) | 37 (2.0) | 0.83 |
| PI chorionic plate | 0.56 (0.27) | 0.52 (0.4) | 0.80 |
| PV chorionic plate (cm/s) | 11 (5.9) | 9 (6.3) | 0.02* |
| PI primary villi | 0.64 (0.22) | 0.65 (0.25) | 0.79 |
| PV primary villi (cm/s) | 11.4 (7.3) | 12.25 (5.5) | 0.27 |
| Abundant secondary villi | 13 (61.9) | 21 (70.0) | 0.55 |
| PI secondary villi | 0.58 (0.13) | 0.68 (0.22) | 0.19 |
| PV secondary villi (cm/s) | 12.7 (8.35) | 10.75 (4.0) | 0.34 |
| Abundant tertiary villi | 8 (38.1) | 14 (46.7) | 0.54 |
| PI tertiary villi | 0.63±0.24 | 0.63±0.17 | 0.48 |
| PV tertiary villi (cm/s) | 9.5 (6.7) | 8.9 (3.1) | 0.46 |
| PI basal plate | 0.49 (0.45) | 0.42 (0.21) | 0.52 |
| PV basal plate (cm/s) | 19.5 (36.9) | 16.3 (18.0) | 0.76 |
Parametric numeric variables are expressed as mean ± SD and non-parametric numeric variables are expressed as median (interquartile range), while qualitative variables are expressed as frequencies and percentages. *P<0.05. Group 1: Normal group; Group 2: FGR group. PI, pulsatile index; PV, peak systolic velocity; SD, standard deviation; FGR, fetal growth restriction.
Discussion
Our aim was to evaluate the diagnostic capacity of SMI Doppler to identify cases with severe placental insufficiency findings. Our results showed that lower values of the PV of the chorionic plate were associated with placental insufficiency, as established by the histologic examination of the placenta. Furthermore, when comparing them with the reference curves published by Sainz et al. for normal pregnancies (15), we can see that the mean value of this variable in our FGR group is distinctly lower than the normal values.
Although research in this field is still scarce, some authors have recently published works applying this technique to FGR cases (21,22). In a recent study, Furuya et al. described the precision of SMI Doppler to identify histologic findings, with the higher precision being for the placental infarction in cases of FGR (23). These results go in line with ours, suggesting that SMI Doppler might be the technique needed to detect insufficient placentas.
According to the models of fetal hypoxia proposed by Kingdom and Kaufmann, FGR at term is caused by intraplacental hypoxia, where a normally oxygenated maternal blood fails to enter fetal circulation due to failed trophoblastic invasion (24). Consecutively, intervillous blood flow is compromised in a heterogeneous way. This might explain why we did not find significant differences when assessing specifically located vessels such as the primary or secondary villi, whereas the chorionic plate reflects the blood flow of larger portions of the placenta.
We consider the novelty of our study is main strength, as it is the first to apply SMI Doppler to the assessment of placental insufficiency in a quantitative way for the identification of histologic findings. However, the study is not without limitations, as we only assess the primary, secondary and tertiary villi of a concrete central part of the placenta. In future studies it should be considered to evaluate different placental areas to evaluate if there are differences between them. Future research in this field might determine the utility of this technique for the identification of FGR cases that are not identified by conventional Doppler, hence contributing to an improved follow-up care of said cases and improve perinatal outcomes.
Conclusions
The PV of the chorionic plate measured with SMI Doppler, have the capacity to identify placental insufficiency findings. Ultrasonographic placental assessment using SMI Doppler appears to be a useful technique for the evaluation of suspected late on-set placental insufficiency.
Acknowledgments
The authors would like to thank the Pathological Anatomy Service of the Hospital de Valme, Seville, Spain for the collaboration in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-807/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-807/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received the approval of the Andalucia’s Board of Biomedicine Ethics Committee (No. 1001-N-18). All patients who agreed to participate gave their written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martins JG, Biggio JR, Abuhamad A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am J Obstet Gynecol 2020;223:B2-B17. [Crossref] [PubMed]
- Lees CC, Marlow N, van Wassenaer-Leemhuis A, Arabin B, Bilardo CM, Brezinka C, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet 2015;385:2162-72. [Crossref] [PubMed]
- Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, Hecher K, Kingdom J, Poon LC, Salomon LJ, Unterscheider J. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 2020;56:298-312. [Crossref] [PubMed]
- Miller J, Turan S, Baschat AA. Fetal growth restriction. Semin Perinatol 2008;32:274-80. [Crossref] [PubMed]
- Figueras F. Unravelling the link among growth restriction, placental disorders, and stillbirth. Paediatr Perinat Epidemiol 2019;33:284-5. [Crossref] [PubMed]
- Paules C, Youssef L, Rovira C, Crovetto F, Nadal A, Peguero A, Figueras F, Eixarch E, Crispi F, Miranda J, Gratacós E. Distinctive patterns of placental lesions in pre-eclampsia vs small-for-gestational age and their association with fetoplacental Doppler. Ultrasound Obstet Gynecol 2019;54:609-16. [Crossref] [PubMed]
- Ganer Herman H, Barber E, Gasnier R, Gindes L, Bar J, Schreiber L, Kovo M. Placental pathology and neonatal outcome in small for gestational age pregnancies with and without abnormal umbilical artery Doppler flow. Eur J Obstet Gynecol Reprod Biol 2018;222:52-6. [Crossref] [PubMed]
- Konje JC, Huppertz B, Bell SC, Taylor DJ, Kaufmann P. 3-dimensional colour power angiography for staging human placental development. Lancet 2003;362:1199-201. [Crossref] [PubMed]
- Mercé LT, Barco MJ, Bau S. Reproducibility of the study of placental vascularization by three-dimensional power Doppler. J Perinat Med 2004;32:228-33. [Crossref] [PubMed]
- Campbell S. Placental vasculature as visualized by 3D power Doppler angiography and 3D color Doppler imaging. Ultrasound Obstet Gynecol 2007;30:917-20. [Crossref] [PubMed]
- Jones NW, Raine-Fenning NJ, Mousa HA, Bradley E, Bugg GJ. Evaluating the intra- and interobserver reliability of three-dimensional ultrasound and power Doppler angiography (3D-PDA) for assessment of placental volume and vascularity in the second trimester of pregnancy. Ultrasound Med Biol 2011;37:376-85. [Crossref] [PubMed]
- Hasegawa J, Suzuki N. SMI for imaging of placental infarction. Placenta 2016;47:96-8. [Crossref] [PubMed]
- Hasegawa J, Yamada H, Kawasaki E, Matsumoto T, Takahashi S, Suzuki N. Application of superb micro-vascular imaging (SMI) in obstetrics. J Matern Fetal Neonatal Med 2018;31:261-3. [Crossref] [PubMed]
- Hata T, Mori N, AboEllail MAM, Ito M, Nitta E, Miyake T, et al. Advances in Color Doppler in Obstetrics. Journal of South Asian Federation of Obstetrics and Gynaecology 2019;11:1-12. [Crossref]
- Sainz JA, Carrera J, Borrero C, García-Mejido JA, Fernández-Palacín A, Robles A, Sosa F, Arroyo E. Study of the Development of Placental Microvascularity by Doppler SMI (Superb Microvascular Imaging): A Reality Today. Ultrasound Med Biol 2020;46:3257-67. [Crossref] [PubMed]
- Sun L, Li N, Jia L, Zhang C, Wang S, Jiao R, Wang L, Ye Y. Comparison of Superb Microvascular Imaging and Conventional Doppler Imaging Techniques for Evaluating Placental Microcirculation: A Prospective Study. Med Sci Monit 2020;26:e926215. [Crossref] [PubMed]
- Garcia-Jimenez R, Garcia-Mejido JA, Valero I, Fernandez-Palacin A, Borrero C, Sainz JA. Intra- and interobserver reliability of superb microvascular imaging (SMI) Doppler for assessing placental microvasculature. Clinical and Experimental Obstetrics & Gynecology 2022;49:177. [Crossref]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol 1985;151:333-7. [Crossref] [PubMed]
- Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 2016;140:698-713. [Crossref] [PubMed]
- Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther 2014;36:117-28. [Crossref] [PubMed]
- Hata T, Kanenishi K, Yamamoto K, AboEllail MAM, Mashima M, Mori N. Microvascular imaging of thick placenta with fetal growth restriction. Ultrasound Obstet Gynecol 2018;51:837-9. [Crossref] [PubMed]
- García-Jiménez R, Arroyo E, Borrero C, Garcia-Mejido JA, Sosa F, Fernández-Palacín A, Sainz JA. Evaluation of Placental Micro-vascularization by Superb Micro-vascular Imaging Doppler in Cases of Intra-uterine Growth Restriction: A First Step. Ultrasound Med Biol 2021;47:1631-6. [Crossref] [PubMed]
- Furuya N, Hasegawa J, Doi M, Koike J, Suzuki N. Accuracy of Prenatal Ultrasound in Evaluating Placental Pathology Using Superb Microvascular Imaging: A Prospective Observation Study. Ultrasound Med Biol 2022;48:27-34. [Crossref] [PubMed]
- Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta 1997;18:613-21; discussion 623-6. [Crossref] [PubMed]


