
Shear wave elastography-based skin assessment system for systemic sclerosis: a supplement or alternative to conventional ultrasound?
Introduction
Systemic sclerosis (SSc) is an immune-mediated rheumatic disease characterized by autoimmunity, widespread tissue fibrosis of the skin and internal organs, and vasculopathic alterations (1). According to the skin involvement evaluated by palpation, SSc can be further subdivided into limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc). There are differences in musculoskeletal and cardiopulmonary involvement between the two subdivisions, which have been previously reported (2-4). Accordingly, assessing skin involvement for a clear classification is an important step in evaluating SSc. The modified Rodnan skin score (mRSS) is the most widely used tool for assessing skin involvement in SSc; however, this method still has several limitations, such as the unsatisfactory interobserver reliability among doctors with different training experience (5,6) and the use of semiquantitative data not sensitive enough for evaluating disease progression or treatment effect in the follow-up (7).
Medical imaging, such as laboratory tests, can provide plenty of quantitative information to reflect anatomical changes and disease progression. Previous studies have found that skin thickness measured by high-frequency ultrasound (HFUS) can reflect site-specific skin involvement (8,9). However, subclinical abnormalities in skin thickness were present in normal mRSS skin in SSc, so traditional results of HFUS can be confusing when evaluating the subtype classification (9-11). In addition, some related studies have found that skin thickness may be affected by sex, body mass index (BMI), age, disease duration, etc., and these influencing factors are interrelated (12,13), thus limiting the application and explainability of HFUS.
Shear wave elastography (SWE), as a new acoustic technique, can be used to quantify the stiffness of tissue according to the physical characteristics of different shear wave propagation speeds in tissues with different mechanical properties (14,15), which expands the evaluation dimension of traditional acoustic technology to the function and structure of the tissue. SWE uses Young’s modulus to measure the stiffness of the target tissue, where a larger value indicates higher stiffness (15). At present, the quantitative assessment of liver cirrhosis by SWE has been applied in the clinic (16). Considering the technical characteristics of SWE, theoretically, SWE can be a suitable imaging evaluation tool for SSc. Our previous studies have confirmed the reliability of SWE in healthy [inter- and intraclass correlation coefficients (ICC): 0.62–0.91] and SSc skin stiffness (ICC: 0.82–0.99) (17-19). Some SWE-based studies on SSc have demonstrated the potential value of SWE in SSc skin assessment and follow-up (7,20,21); however, evidence based on the current research still fails to answer the following questions: (I) can skin thickness and stiffness at multiple sites throughout the body distinguish dcSSc and lcSSc; (II) is the skin thickness and stiffness at every site equally affected by the clinical characteristics such as disease duration, or are their differences between sites; and (III) is SWE a choice to HFUS or is it just a supplement. Aiming to address these issues, we designed this cross-sectional study that included dcSSc and lcSSc patients whose skin at 17 sites was assessed by palpation, HFUS, and SWE; additionally, the mRSS, skin thickness, and Young’s modulus were recorded.
Methods
Study participants
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of West China Hospital, Sichuan University [approval number: 2018(210)], and informed consent was obtained from all participants. A total of 100 SSc patients from the outpatient clinic who met the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2013 criteria for SSc (22) or LeRoy’s criteria for the classification of early SSc (23) were included between September 2018 and May 2022. Age, sex, BMI, disease duration, and subdivisions of skin involvement (dcSSc and lcSSc) were evaluated and recorded. Skin involvement was scored according to the mRSS over 17 anatomical sites (24) by an experienced dermatologist trained at the European League Against Rheumatism Scleroderma Trials and Research group course with more than 10 years of experience in mRSS (25). The evaluator was blinded to the ultrasound results.
Ultrasonography assessments
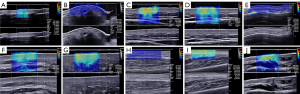
Ultrasonography assessments were performed before starting treatment. All patients were instructed not to perform any exercise two hours before the examination and to rest completely for 5 minutes before the examination. The room temperature was constant at 25 ℃. One trained sonographer with 8 years of experience in musculoskeletal ultrasonography and 3 years in SWE conducted all examinations on an Aixplorer ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) with an SL 15-4 multifrequency linear probe. The instrument settings and scanning process were consistent with a previous study (18). Skin thickness (epidermis and dermis) was measured in 2D mode, and skin stiffness (dermis) was measured in SWE mode. The 17 selected target sites were consistent with those used in the study of Moore et al. (24); standard images are shown in Figure 1. For each site, three consecutive values were measured, and the average value was assessed; the results were expressed in millimeters (mm) for skin thickness or kilopascals (kPa) for skin stiffness. During the scanning process, the sonographer was unaware of the mRSS results.
Statistical analysis
Statistical analyses were performed using SPSS 24.0 software (IBM, Armonk, NY, USA) and MedCalc software 20.0.4 (MedCalc Software Ltd., Ostend, Belgium). Continuous variables that conformed to a normal distribution were described as the means ± standard deviations, and a t-test was used to assess differences between the two groups. For other continuous variables, the median (25th percentile to 75th percentile) was used, and a Mann-Whitney U test was applied to compare two groups in the case of nonnormally distributed continuous variables. The chi-square test was used to compare sex proportions in different groups. Logistic regression with the entering method was used to obtain the response probability to dcSSc and lcSSc of skin thickness, stiffness, and the combination of the two. Further receiver operating characteristic (ROC) analysis and area under the curve (AUC) were used to evaluate the thickness and stiffness classification effect on dcSSc and lcSSc. A Z test was used to compare ROC curves. Multiple linear regression with a stepwise method was used to screen for independent influencing factors affecting skin thickness and stiffness (excluding parameters with P values over 0.10). In regression analysis, the gender variable was given a value of 0 for men and 1 for women. In the abovementioned analysis, the thickness or stiffness at bilaterally symmetrical sites was expressed as the average of the respective results on both sides. For the comparison of skin thickness and stiffness between different mRSS scores at different sites, the Kruskal-Wallis test and further multiple comparisons were performed in the stepwise step-down method. The thickness or stiffness at 17 target sites was treated as the independent variable in the analysis. A P value of <0.05 indicated statistical significance for two-sided tests.
Results
A total of 49 lcSSc and 51 dcSSc patients were included in this study. Their demographic characteristics and clinical features are compared in Table 1. There was no significant difference in age, BMI, disease duration, or Raynaud’s phenomenon between lcSSc and dcSSc, and female patients were the majority. Additionally, the proportion of women in the lcSSc group was significantly higher than that in the dcSSc group (85.7% vs. 64.7%, P=0.015). The total mRSSs were higher in dcSSc than in lcSSc (23 vs. 8, P<0.001).
Table 1
| Characteristics | lcSSc (n=49) | dcSSc (n=51) | P |
|---|---|---|---|
| Gender (male:female) | 7:42 | 18:33 | 0.015 |
| Age (years), median [IQR] | 47.0 [39.0–51.5] | 48.0 [35.0–59.0] | 0.530 |
| BMI (kg/m2), median [IQR] | 22.6 [20.1–24.6] | 22.3 [19.3–23.5] | 0.172 |
| Duration (years), median [IQR] | 3.0 [1.0–10.0] | 2.0 [1.0–7.0] | 0.140 |
| Raynaud’s phenomenon (+:−) | 45:4 | 42:9 | 0.266 |
| Total mRSS, median [IQR] | 8 [3–13] | 23 [12–33] | <0.001 |
SSc, systemic sclerosis; lcSSc, limited SSc; dcSSc, diffused SSc; BMI, body mass index; mRSS, modified Rodnan skin score; IQR, interquartile range.
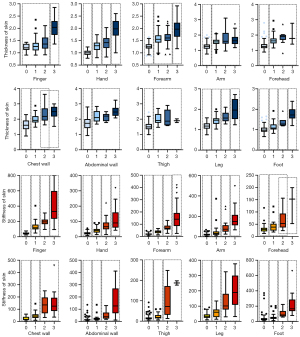
For skin stiffness, dcSSc had a higher Young’s modulus than lcSSc at most sites except the finger, foot, and forehead. With reference to thickness, dcSSc had a thicker skin layer than lcSSc at most sites, except for the finger. The detailed comparison is shown in Table 2.
Table 2
| Stiffness and thickness of target sites | lcSSc (n=49), median (IQR) | dcSSc (n=51), median (IQR) | P |
|---|---|---|---|
| Stiffness (kPa) | |||
| Finger | 222.3 (153.2–295.3) | 211.1 (130.3–408.2) | 0.959 |
| Hand | 38.7 (25.5–52) | 63.6 (32.7–105.2) | 0.006 |
| Forearm | 25.5 (17.2–39.7) | 58.4 (33.8–110.9) | <0.001 |
| Arm | 20.3 (13.5–27.5) | 51 (23.2–103.4) | <0.001 |
| Thigh | 13.5 (9.9–17.4) | 19.5 (13.4–32.4) | 0.001 |
| Leg | 36.9 (22.2–49.9) | 50.3 (30.7–76.6) | 0.004 |
| Foot | 33.7 (20.4–45.5) | 41.4 (21–75.9) | 0.168 |
| Forehead | 29.9 (21.2–39.1) | 33.5 (23.8–52.5) | 0.130 |
| Chest wall | 15.2 (12.1–31.2) | 48.2 (31.8–95.9) | <0.001 |
| Abdominal wall | 12.9 (10.4–16.2) | 25.8 (13.7–72.9) | <0.001 |
| Thickness (mm) | |||
| Finger | 1.4 (1.3–2.1) | 1.7 (1.4–2.1) | 0.221 |
| Hand | 1.1 (1.0–1.3) | 1.4 (1.2–1.7) | <0.001 |
| Forearm | 1.3 (1.2–1.5) | 1.6 (1.5–2.0) | <0.001 |
| Arm | 1.2 (1.1–1.3) | 1.5 (1.4–1.8) | <0.001 |
| Thigh | 1.4 (1.3–1.7) | 1.7 (1.6–2.0) | <0.001 |
| Leg | 1.2 (1.1–1.4) | 1.4 (1.2–1.6) | 0.001 |
| Foot | 1.0 (0.9–1.1) | 1.1 (1.0–1.3) | 0.028 |
| Forehead | 1.3 (1.2–1.5) | 1.5 (1.3–1.9) | 0.004 |
| Chest wall | 1.6 (1.4–1.9) | 2.0 (1.8–2.3) | <0.001 |
| Abdominal wall | 1.7 (1.4–2.0) | 2.1 (1.7–2.4) | 0.001 |
SSc, systemic sclerosis; lcSSc, limited SSc; dcSSc, diffused SSc.
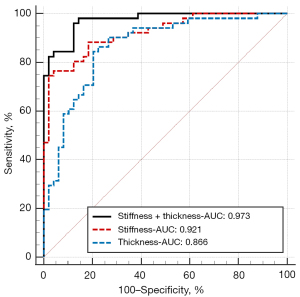
To study the classification effect of skin thickness and stiffness on lcSSc and dcSSc, we plotted the ROC curves of the response probability in logistic regression, as shown in Figure 2. The AUC of skin thickness alone was 0.866 (0.783–0.926), and the AUC of skin stiffness alone was 0.921 (0.850–0.966), while in the current sample, there was no significant difference between the two curves (P=0.205). Correspondingly, the combination of thickness and stiffness had the highest classification effect, with an AUC of 0.973 (0.919–0.995); the observed difference was statistically significant (combination vs. stiffness, P=0.002, combination vs. thickness, P=0.021).
Next, to show the effect of disease duration, sex, BMI, and age on the skin thickness and stiffness of each site in lcSSc and dcSSc, we made a heatmap of the results of the standardized coefficient beta in the multiple regression (Figure 3). Overall, the following two regularities could be observed: first, skin stiffness was less affected by clinical characteristics than skin thickness; second, skin thickness and stiffness of dcSSc were more affected by clinical characteristics than lcSSc. For skin thickness, longer disease duration was associated with a thinner skin layer on the forearm, arm, chest wall, abdominal wall, and thigh in lcSSc, including the leg in dcSSc. The only exception was the finger, as the skin thickness of the finger tended to be thicker in patients with longer disease duration. BMI was an insignificant factor, and no significant influence on skin thickness was found at all target sites throughout the body. Female sex and younger age were related to a thinner skin layer at some sites. For skin stiffness, longer disease duration was associated with greater skin stiffness at the finger in lcSSc and at the finger, hand, and forehead in dcSSc. Female sex, greater BMI, and younger age were associated with lower skin Young’s modulus at some sites, but there did not seem to be any apparent regularity in the sites affected by these factors.

Finally, we compared the skin thickness and stiffness between different mRSSs at each site, as shown in Figure 4. Differences between multiple groups were significant (all P<0.01), and in the following multiple comparisons, there were no significant differences between the subgroups in each dashed box. Skin stiffness could achieve greater discrimination between different mRSSs at multiple sites, such as the finger and arm, than skin thickness.
Discussion
Although there were no significant differences in age, BMI, or disease duration among recruited SSc patients, the sex ratio in lcSSc and dcSSc was different. The female sex accounted for 85.7% of lcSSc and 64.7% of dcSSc. In previous studies, no strong evidence supported sex differences in lcSSc and dcSSc. This result may be due to bias because this was a cross-sectional study. Interestingly, this sex ratio was similar to that reported in a retrospective observational study, which included 375 SSc patients (26), i.e., 81% female patients in lcSSc and 60% female patients in dcSSc. Whether sex is associated with different subdivisions of skin involvement needs to be further confirmed by future epidemiological research.
With reference to skin thickness, Sulli et al. (11) found that in patients classified as lcSSc, the thickened skin layer could be detected by ultrasound in skin areas with a normal mRSS, such as the arm, chest, or abdominal wall. Our results further showed that dcSSc had thicker skin than lcSSc throughout the body, except for fingers, similar to a previous study by Hesselstrand et al. (8) compared the skin thickness at the finger, hand, forearm, chest wall, and leg in dcSSc and lcSSc, finding that dcSSc had greater skin thickness, except for the finger. With reference to skin stiffness, dcSSc had a higher Young’s modulus than lcSSc at most sites except for the finger, foot, and forehead. After combining all sites using logistic regression, we found that skin thickness, skin stiffness, or a combination of the two had good diagnostic accuracy in distinguishing lcSSc from dcSSc. Although a significant difference was not observed in the statistical comparison between the stiffness and thickness curves at a small sample size, it is expected from the curve that as the sample size increases, the stiffness may achieve a higher classification effect than skin thickness. However, at the same time, we observed that the classification effect could be further improved after combining stiffness with thickness.
Some commonalities and exceptions were observed in the following exploration of influencing factors. We found that thinner skin thickness was associated with longer disease duration. Since disease progression goes from the edematous phase to the atrophic phase, a longer disease duration suggests that patients are more likely to be in the fibrotic or atrophic phase, reflected in the thinner skin layer. We also observed that the finger was a special site where longer disease duration was associated with thicker skin thickness in dcSSc. After following up dcSSc and lcSSc patients for 4 years, Akesson et al. (27) found a difference in the duration of the edematous phase at different sites. Additionally, the changes in skin thickness during follow-up between dcSSc and lcSSc patients also had differences, which may explain the differences in the effect of disease duration at different sites in our study. As reported in many studies, the effect of age and sex on skin thickness is controversial (27,28). Physiologically, women may have thinner skin, and with age, the skin first thickens and then remains stable, after which it becomes thinner in old age, while the correlations vary across the skin at different sites. While our results are consistent with gender differences in previous studies, older age may be associated with thicker skin in SSc patients. Due to the lack of older adults in the population included in this study, the effects of aging on the skin may be underestimated, and there is a lack of evidence supporting the relationship between skin thickness and age in pathological conditions. Considering skin stiffness, we found it was less affected by clinical features. Longer disease duration was associated with greater skin stiffness at the finger in lcSSc and that at the finger, hand, and forehead in dcSSc, while female sex, greater BMI, and younger age were associated with lowering skin Young’s modulus at some sites. Previous studies have found that “bone-proximity” hardening artifacts may lead to higher elasticity measurements (29). As a result, lower BMI may result in a thinner subcutaneous fat layer, affecting Young’s modulus values of the skin at some sites. In some studies on healthy populations, women were found to have lower Young’s modulus values of the skin (19), which is consistent with the present study’s findings. Although the effect of age on skin stiffness is also controversial (7,19), our results showed that age had a very limited effect on skin stiffness at most sites.
Finally, the comparison with mRSS also suggested that Young’s modulus had a stronger effect size than skin thickness. With the same sample size and distribution, skin stiffness showed better discrimination than the thickness in distinguishing different mRSSs at target sites, suggesting that it could detect lesions of the target site with higher sensitivity.
It is worth noting that skin is a layered material, and the shear wavelength (1–a few mm) applied in our study was larger than or equal to the skin thickness (1–2.4 mm). In this case, the Lamb wave propagation effect will occur theoretically. Based on this, Mo et al. found that the stiffness of thin-layer gelatin-agar phantoms measured by SWE was lower in thinner samples (30). Nevertheless, Gennisson et al. reported that skin stiffness was not influenced by skin thickness, consistent with our previous study (19,31). Therefore, in healthy and diseased skin in vivo, whether the skin of different thicknesses will show a different mechanical modulus in SWE due to the Lamb wave propagation effect needs to be further studied. On the other hand, skin is a mechanically anisotropic organ. However, Piérard and Lapière found that collagen bundles and elastic fibers exhibited an evident indifference to the nature and number of fibers oriented in any direction in the normal human skin (32). Some studies have suggested that skin stiffness in different directions at the same site is not significantly different (17,33). Whether the skin anisotropy properties will be reflected in SWE remains controversial.
The limitations of this study include the following. First, the work is a cross-sectional study; accordingly, there may be some bias in the inclusion of subjects, and some patients with mild symptoms because of not seeking medical attention and borderline patients may not be included. The bias will lead to lower sensitivity/specificity in clinical practice for new/borderline diagnoses. Second, combined ultrasonographic assessment of skin thickness and stiffness at 17 sites is time-consuming (approximately 20 minutes for each assessment). The screening of sites and indicators with the best evaluation value will be our next research focus. Third, although the sonographer was unaware of the mRSS results, it was not blinded because most SSc patients have obvious diseases. Fourth, measurements of skin stiffness may be biased because of the inevitable use of ultrasound gel and slight compression. Whether the weight of the gel, which may cause skin deformation due to skin as the most superficial structure, or the different cutaneous absorption of gel by the diseased skin may affect the skin mechanical modulus. Probe compression can produce a nonlinear tissue response such that the skin stiffness is overestimated, so we applied an operator with 3 years of SWE experience to perform skin examination in our study and minimize this human error as much as possible. Finally, our study used a 4–15 MHz probe because our machine was not equipped with higher frequency probes. Higher frequency was able to show the finer structure of the skin. Our frequency applied was sufficient for skin thickness assessment because SSc was mainly hyperplasia of collagen fibers in the dermis, which caused changes in skin thickness.
Conclusions
For the skin assessment in SSc, the SWE-based assessment system showed several advantages over skin thickness assessment, including less influence of clinical features and greater sensitivity to discriminate different mRSSs. When combined with the results of HFUS, the diagnostic value of SWE can be further improved. In the field of SSc skin assessment, SWE has the potential to become a primary imaging assessment tool as well as conventional ultrasound.
Acknowledgments
Funding: This study was financially sponsored by the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2020HXFH001); the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Z2021LC002); and the National Natural Science Foundation of China (No. 82272003).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1267/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of West China Hospital, Sichuan University [No. 2018(210)], and informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685-99. [Crossref] [PubMed]
- Foeldvari I, Klotsche J, Kasapcopur O, Adrovic A, Terreri MT, Sakamoto AP, et al. Differences Sustained Between Diffuse and Limited Forms of Juvenile Systemic Sclerosis in an Expanded International Cohort. Arthritis Care Res (Hoboken) 2022;74:1575-84. [Crossref] [PubMed]
- Hubac J, Gilson M, Gaudin P, Clay M, Imbert B, Carpentier P. Ultrasound prevalence of wrist, hand, ankle and foot synovitis and tenosynovitis in systemic sclerosis, and relationship with disease features and hand disability. Joint Bone Spine 2020;87:229-33. [Crossref] [PubMed]
- Karalilova R, Kazakova M, Sapundzhieva T, Dichev V, Batalov Z, Sarafian V, Batalov A. Serum YKL-40 and IL-6 levels correlate with ultrasound findings of articular and periarticular involvement in patients with systemic sclerosis. Rheumatol Int 2019;39:1841-8. [Crossref] [PubMed]
- Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, Weinstein A, Weisman M, Mayes M, Collier D. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995;22:1281-5. [PubMed]
- Park JW, Ahn GY, Kim JW, Park ES, Kang JH, Chang SH, Choi IA, Yoo SJ, Park JK, Shin K, Park YB, Jun JB, Czirják L, Allanore Y, Matucci-Cerinic M, Lee EB. Impact of EUSTAR standardized training on accuracy of modified Rodnan skin score in patients with systemic sclerosis. Int J Rheum Dis 2019;22:96-102. [Crossref] [PubMed]
- Santiago T, Santiago M, Coutinho M, Salvador MJ, Da Silva JAP. How much of skin improvement over time in systemic sclerosis is due to normal ageing? A prospective study with shear-wave elastography. Arthritis Res Ther 2020;22:50. [Crossref] [PubMed]
- Hesselstrand R, Scheja A, Wildt M, Akesson A. High-frequency ultrasound of skin involvement in systemic sclerosis reflects oedema, extension and severity in early disease. Rheumatology (Oxford) 2008;47:84-7. [Crossref] [PubMed]
- Li H, Furst DE, Jin H, Sun C, Wang X, Yang L, He J, Wang Y, Liu A. High-frequency ultrasound of the skin in systemic sclerosis: an exploratory study to examine correlation with disease activity and to define the minimally detectable difference. Arthritis Res Ther 2018;20:181. [Crossref] [PubMed]
- Flower VA, Barratt SL, Hart DJ, Mackenzie AB, Shipley JA, Ward SG, Pauling JD. High-frequency Ultrasound Assessment of Systemic Sclerosis Skin Involvement: Intraobserver Repeatability and Relationship With Clinician Assessment and Dermal Collagen Content. J Rheumatol 2021;48:867-76. [Crossref] [PubMed]
- Sulli A, Ruaro B, Smith V, Paolino S, Pizzorni C, Pesce G, Cutolo M. Subclinical dermal involvement is detectable by high frequency ultrasound even in patients with limited cutaneous systemic sclerosis. Arthritis Res Ther 2017;19:61. [Crossref] [PubMed]
- Meng Y, Feng L, Shan J, Yuan Z, Jin L. Application of high-frequency ultrasound to assess facial skin thickness in association with gender, age, and BMI in healthy adults. BMC Med Imaging 2022;22:113. [Crossref] [PubMed]
- Van Mulder TJ, de Koeijer M, Theeten H, Willems D, Van Damme P, Demolder M, De Meyer G, Beyers KC, Vankerckhoven V. High frequency ultrasound to assess skin thickness in healthy adults. Vaccine 2017;35:1810-5. [Crossref] [PubMed]
- Ryu J, Jeong WK. Current status of musculoskeletal application of shear wave elastography. Ultrasonography 2017;36:185-97. [Crossref] [PubMed]
- Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991;13:111-34. [Crossref] [PubMed]
- Zhou X, Rao J, Wu X, Deng R, Ma Y. Comparison of 2-D Shear Wave Elastography and Point Shear Wave Elastography for Assessing Liver Fibrosis. Ultrasound Med Biol 2021;47:408-27. [Crossref] [PubMed]
- Xiang X, Yan F, Yang Y, Tang Y, Wang L, Zeng J, Qiu L. Quantitative Assessment of Healthy Skin Elasticity: Reliability and Feasibility of Shear Wave Elastography. Ultrasound Med Biol 2017;43:445-52. [Crossref] [PubMed]
- Yang Y, Qiu L, Wang L, Xiang X, Tang Y, Li H, Yan F. Quantitative Assessment of Skin Stiffness Using Ultrasound Shear Wave Elastography in Systemic Sclerosis. Ultrasound Med Biol 2019;45:902-12. [Crossref] [PubMed]
- Yang Y, Wang L, Yan F, Xiang X, Tang Y, Zhang L, Liu J, Qiu L. Determination of Normal Skin Elasticity by Using Real-time Shear Wave Elastography. J Ultrasound Med 2018;37:2507-16. [Crossref] [PubMed]
- Hou Y, Zhu QL, Liu H, Jiang YX, Wang L, Xu D, Li MT, Zeng XF, Zhang FC. A preliminary study of acoustic radiation force impulse quantification for the assessment of skin in diffuse cutaneous systemic sclerosis. J Rheumatol 2015;42:449-55. [Crossref] [PubMed]
- Liu H, Hou Y, Zhu QL, Xu D, Wang L, Li JC, Jiang YX, Wang Q, Li MT, Zhang FC, Zeng XF. A preliminary study of skin ultrasound in diffuse cutaneous systemic sclerosis: Does skin echogenicity matter? PLoS One 2017;12:e0174481. [Crossref] [PubMed]
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747-55. [Crossref] [PubMed]
- LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573-6. [PubMed]
- Moore TL, Lunt M, McManus B, Anderson ME, Herrick AL. Seventeen-point dermal ultrasound scoring system--a reliable measure of skin thickness in patients with systemic sclerosis. Rheumatology (Oxford) 2003;42:1559-63. [Crossref] [PubMed]
- Tyndall A, Ladner UM, Matucci-Cerinic M. The EULAR Scleroderma Trials and Research Group (EUSTAR): an international framework for accelerating scleroderma research. Curr Opin Rheumatol 2008;20:703-6. [Crossref] [PubMed]
- De Almeida Chaves S, Porel T, Mounié M, Alric L, Astudillo L, Huart A, Lairez O, Michaud M, Prévot G, Ribes D, Sailler L, Gaches F, Adoue D, Pugnet G. Sine scleroderma, limited cutaneous, and diffused cutaneous systemic sclerosis survival and predictors of mortality. Arthritis Res Ther 2021;23:295. [Crossref] [PubMed]
- Akesson A, Hesselstrand R, Scheja A, Wildt M. Longitudinal development of skin involvement and reliability of high frequency ultrasound in systemic sclerosis. Ann Rheum Dis 2004;63:791-6. [Crossref] [PubMed]
- Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol 2005;11:221-35. [Crossref] [PubMed]
- Bortolotto C, Turpini E, Felisaz P, Fresilli D, Fiorina I, Raciti MV, Belloni E, Bottinelli O, Cantisani V, Calliada F. Median nerve evaluation by shear wave elastosonography: impact of "bone-proximity" hardening artifacts and inter-observer agreement. J Ultrasound 2017;20:293-9. [Crossref] [PubMed]
- Mo J, Xu H, Qiang B, Giambini H, Kinnick R, An KN, Chen S, Luo Z. Bias of shear wave elasticity measurements in thin layer samples and a simple correction strategy. Springerplus 2016;5:1341. [Crossref] [PubMed]
- Gennisson JL, Baldeweck T, Tanter M, Catheline S, Fink M, Sandrin L, Cornillon C, Querleux B. Assessment of elastic parameters of human skin using dynamic elastography. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:980-9. [Crossref] [PubMed]
- Piérard GE, Lapière CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer's lines. Am J Dermatopathol 1987;9:219-24. [Crossref] [PubMed]
- Luo CC, Qian LX, Li GY, Jiang Y, Liang S, Cao Y. Determining the in vivo elastic properties of dermis layer of human skin using the supersonic shear imaging technique and inverse analysis. Med Phys 2015;42:4106-15. [Crossref] [PubMed]


