
Primary mediastinal hepatoid adenocarcinoma: case description of a rare condition
Introduction
Hepatoid adenocarcinoma (HAC) is a rare extrahepatic tumor with hepatocellular carcinoma-like structure and cytologic features, resembling hepatocellular carcinoma on histopathology and immunohistochemistry. HAC occurs in extrahepatic organs or tissue and is often associated with a significant increase in serum alpha-fetoprotein (AFP). HAC can occur in the gastrointestinal tract, ovaries, lungs, gallbladder, pancreas, uterus, and bladder but rarely in the mediastinum (1-3). In this article, we present a case of HAC in the mediastinum and provide a concise review of this malignancy.
Case presentation
A 44-year-old man presenting with unintended weight loss over the past few months underwent a chest computed tomography (CT) and a mass was incidentally discovered. Contrast-enhanced chest CT was arranged to further determine the characteristics of the mass. He denied any relevant medical history or symptoms such as cough or chest pain. Laboratory tests showed a significantly elevated AFP level (>1,000 ng/mL), but no other lab values were observed.
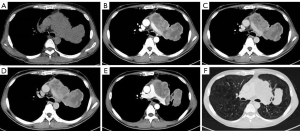
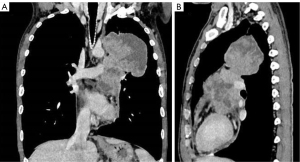
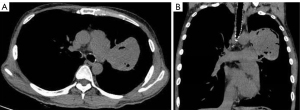
The plain and contrast-enhanced chest CT scan (Figure 1) revealed a large irregular soft tissue mass in the mid-mediastinum. The mass protruded upward into the left upper lobe (LUL) and downward into the pericardial cavity in a mushroom shape (Figure 2) with clear boundaries. The size of the lesion was approximately 9.2 cm × 8.1 cm × 12.4 cm. The plain CT demonstrated slightly heterogeneous attenuation of the mass with solid (45 Hounsfield Unit, HU) and less dense components. The contrast-enhanced scan showed slightly heterogeneous enhancement of the mass with areas of necrosis. Multiple enlarged lymph nodes were seen in the adjacent mediastinum. The lesion compressed the pulmonary trunk and the left pulmonary artery. The left superior lobar bronchus showed segmental occlusion. A malignant mediastinal mass invading the upper lobe of the left lung was diagnosed. To present this case in more detail, we provide the plain and venous phase CT images of this patient (Videos 1-4).
Fiberoptic bronchoscopy showed a cauliflower-like neoplasm at the opening of the left upper lobar bronchus. The anterior and postero-apical segments of the bronchus opening showed external compressive stenosis. The left mainstem bronchus and the rest of the left bronchial tree were unobstructed.
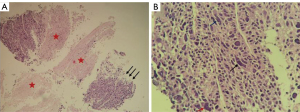
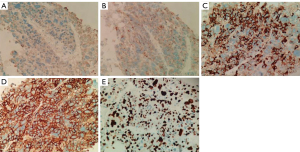
To further confirm the tumor characteristics, he underwent a percutaneous needle biopsy of the left lung under CT guidance. Histologically (Figure 3), the tumor cells were arranged in nested clusters with large necrotic areas. The tumor cells had a polygonal shape with abundant cytoplasm, eosinophilia, distinct nucleoli, multinucleated cells, and odd cells, and the interstitium was rich in blood sinusoids (Figure 3). Immunohistochemically (Figure 4), the tumor cells were weakly positive for cytokeratin (CK) and synaptophysin (Syn), partially and strongly positive for chromogranin A (CGA), and diffusely and strongly positive for glypican-3 (Gly-3). The Ki67 proliferation index was approximately 50%. Pathological microscopic examination and immunohistochemical features supported the diagnosis of HAC.
Gastrointestinal endoscopy showed no abnormalities. Additionally, no other primary tumors were found on CT examination of the whole abdomen. The patient was treated with paclitaxel plus cisplatin (TP regimen) combined with targeted therapy (Avastin plus Atilizumab). While admitted to the hospital for the third cycle of treatment, the patient had a follow-up chest CT that showed that the mediastinal mass had shrunk (Figure 5). After completion of three cycles of treatment, the patient’s serum AFP level decreased to 98.1 ng/mL. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
HAC is an uncommon malignant tumor with morphologic features similar to hepatocellular carcinoma. Ishikura et al. reported the first case of HAC of the stomach in 1985 (1), followed by the first case of HAC of the lung in 1990 (2). In 2010, Metzgeroth (3) concluded that the most common locations of HAC were, in descending order, the stomach (63%), ovary (10%), lung (5%), gallbladder (4%), pancreas (4%), and uterus (4%). HAC may also occur rarely in organs such as the prostate (4). This may be related to the fact that the above organs are embryological derivatives of the primitive forestomach. As such, this type of adenocarcinoma tends to differentiate toward hepatocytes and therefore tends to produce AFP (5). HAC occurs more commonly in middle-aged and elderly people, especially males.
HAC of the mediastinum is very rare. A search of PubMed and Web of Science yielded only five articles, listed in Table 1.
Table 1
| Author | Year | Gender | Age | Chief complaints | Smoker | Serum AFP (ng/mL) | Size (cm) | Location | Enhancement |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. (6) | 2022 | M | 53y | Front chest pain lasting 6 months | Yes | Negative | 5.9×3.9×2.6 (the largest of three masses) | Anterior mediastinum | Slightly inhomogeneous enhancement |
| Jeong et al. (7) | 2021 | M | 53y | Occasionally detected | Not given | Negative | 6 | Anterior mediastinum | Slightly inhomogeneous enhancement |
| Hu et al. (8) | 2016 | M | 43y | Incidentally found on a routine chest radiograph | Not given | 800 | 6.5 | Anterior mediastinum | Slightly inhomogeneous enhancement |
| Hu et al. (9) | 2015 | M | 28y | Chest and shoulder pain | No | 155,278.00 | 18 | Anterior mediastinum | Slightly inhomogeneous enhancement |
| Franke et al. (10) | 2004 | F | 70y | Respiratory distress | Not given | Negative | 18×12×8 | Anterior mediastinum (thymic region) | Without contrast-enhanced |
HAC, hepatoid adenocarcinoma; AFP, alpha-fetoprotein; M, male; F, female.
Based on the case in this article and combined with previous literature reports, the imaging manifestation is summarized as follows. Mediastinal HAC usually appears on CT as a single giant mass in the anterior/middle mediastinum. In a few cases, it may present as a cluster of multiple masses appearing with a maximum diameter greater than 6 cm with clear boundaries, heterogenous density, and slightly heterogenous contrast enhancement on enhanced scans. In our case, the mass was very large and protruded upward into the LUL and downward into the pericardial cavity in a mushroom shape. The bronchus of the LUL was narrowed by compression. However, the interface between the mass and the lung was clear, and no pulmonary atelectasis was observed. Because of the large size of mediastinal HAC masses, it is sometimes difficult to localize the mass (whether originating in the mediastinum or lung) with preoperative imaging alone. Additionally, because of the advanced clinical stage and difficulty of surgery when these tumors are detected, accurate postoperative localization is often lacking. According to previous reports, mediastinal HAC is less common than pulmonary HAC. The enhancement of mediastinal HAC is primarily slightly heterogeneous, unlike gastric HAC, which shows moderately heterogeneous enhancement (11). In general, mediastinal HAC does not show the “fast-in, fast-out” enhancement of hepatocellular carcinoma, nor does it resemble the progressive and marked enhancement of adenocarcinoma.
The histopathological morphological features of HAC often consist of two distinct but closely related adenocarcinomatous regions and hepatoid differentiation regions similar to hepatocellular carcinoma. Thus, the presence of hepatoid differentiation zones is the reason that some patients present with elevated AFP, as in our case. Inagawa et al. (12) suggested that serum AFP levels were related to the degree of tumor differentiation. Some patients have normal serum AFP levels, indicating a low degree of differentiation. However, serum AFP levels are not necessary to confirm the diagnosis of HAC. Nagai et al. (13) concluded that despite elevated AFP levels, HAC can be diagnosed if hepatoid differentiation is present on pathologic examination.
Mediastinal HAC must be distinguished from mediastinal yolk cyst tumors, mediastinum-type lung cancer, lymphoma, and invasive thymoma. Mediastinal yolk tumors occur more commonly in young men. Over 90% of patients with yolk tumors have markedly elevated AFP. The mass usually occurs in the anterior superior mediastinum with unclear borders and heterogeneous density and may present with cystic changes, necrosis, and calcification. The CT enhancement pattern is multiple strips and lines with lamellar nonenhanced areas, with mild to moderate enhancement. Mediastinum-type lung cancer tends to occur in adult men over 40 years of age with a history of smoking. Additionally, patients with mediastinum-type lung cancer do not present with elevated AFP. Lymphoma is mostly located in the anterior and middle mediastinum, growing across the midline. The mass is often large with multiple nodular fusion changes and is mostly uniform soft tissue density with light to moderate enhancement. Invasive thymomas are commonly located in the anterior mediastinum and manifest as lobulated soft tissue masses with uneven density and irregular margins. The fat space with the adjacent heart and vasculature is always indistinct or disappears. Most of it is significantly inhomogeneous enhancement with necrosis and cysts in the center part.
In summary, mediastinal HAC should be considered in patients presenting with a large mediastinal mass with clear borders and necrosis with mild enhancement and markedly elevated AFP. Final diagnosis should be made by pathology.
Acknowledgments
Funding: This work was supported by the Key Project of Science and Technology Program of Jiangxi Provincial Department of Education (No. GJJ200106) and the Applied Research Cultivation Program of Jiangxi Provincial Department of Science and Technology (No. 20212BAG70048).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1124/coif). The authors report that this work was supported by the Key Project of Science and Technology Program of Jiangxi Provincial Department of Education (No. GJJ200106) and the Applied Research Cultivation Program of Jiangxi Provincial Department of Science and Technology (No. 20212BAG70048). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer 1985;56:840-8. [Crossref] [PubMed]
- Ishikura H, Kanda M, Ito M, Nosaka K, Mizuno K. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol 1990;417:73-80. [Crossref] [PubMed]
- Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie 2010;33:263-9. [Crossref] [PubMed]
- Ayala Soriano C, Benitez Barzaga M, Chhina A, Jain M, Nava VE. Hepatoid prostatic carcinoma with adrenal metastasis and novel genetic alterations. Diagn Cytopathol 2022;50:E310-E314. [Crossref] [PubMed]
- Akiyama S, Tamura G, Endoh Y, Fukushima N, Ichihara Y, Aizawa K, Kawata S, Motoyama T. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer 2003;106:510-5. [Crossref] [PubMed]
- Zhang G, Wen C, Chen B, Dai H, Lin R, Huang Y, Xiang X. Mediastinal Hepatoid Adenocarcinoma Treated With Arterial Interventional Therapy: A Case Report and Review of Literature. Front Oncol 2022;12:785888. [Crossref] [PubMed]
- Jeong JS, Kang HJ, Jo U, Song MJ, Nam SY, Song JS. Hepatoid thymic carcinoma: a case report of a rare subtype of thymic carcinoma. J Pathol Transl Med 2021;55:230-4. [Crossref] [PubMed]
- Hu N, Tan Y, Luo J, Cheng Z, Wang Y. 18F-FDG PET/CT of Primary Mediastinal Hepatoid Adenocarcinoma. Clin Nucl Med 2016;41:321-2. [Crossref] [PubMed]
- Hu CH, Li QL, Li HP, Fan SQ, Zhang HX, Liu XL, He Y, Huang M, Lu M, Wang SS, Wu F. Rare coexistence of mediastinal hepatoid adenocarcinoma, idiopathic azoospermia and horseshoe kidney: a case report and review of the literature. Int J Clin Exp Pathol 2015;8:11741-6. [PubMed]
- Franke A, Ströbel P, Fackeldey V, Schäfer R, Göller T, Becker HP, Schöneich R, Müller-Hermelink HK, Marx A. Hepatoid thymic carcinoma: report of a case. Am J Surg Pathol 2004;28:250-6. [Crossref] [PubMed]
- Huang WP, Li LM, Li J, Yuan JH, Hou P, Liu CC, Ma YH, Liu XN, Han YJ, Liang P, Gao JB. Computed Tomography Features and Clinical Prognostic Characteristics of Hepatoid Adenocarcinoma of the Stomach. Front Oncol 2021;11:772636. [Crossref] [PubMed]
- Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, Fukao K, Itabashi M. Hepatoid adenocarcinoma of the stomach. Gastric Cancer 2001;4:43-52. [Crossref] [PubMed]
- Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer 1993;72:1827-35. [Crossref] [PubMed]


