
Effective clinical response of lung adenocarcinoma harboring EGFR 19Del/T790M/in cis-C797S osimertinib to osimertinib and gefitinib combination therapy
Introduction
Cancer is one of the leading causes of death in the world and a significant factor limiting life expectancy (1). Among them, the incidence of lung cancer is second only to the first, representing approximately 2.2 million (11.4%) newly diagnosed cancers cases and 1.8 million (18.0%) deaths (2). Non-small cell lung cancer (NSCLC) accounts for ~85% of all lung cancer cases (3). Thus, there is immense research interest in studying functional genes underlying development of NSCLC.
Oncogene activation is a critical step towards the development of NSCLC, particularly lung adenocarcinoma (LADC). These activated genes are referred to as driver oncogenes (4,5), and include epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1, receptor tyrosine kinase (ROS1), B-Raf proto-oncogene serine/threonine protein kinase (BRAF), mesenchymal epithelial transition factor receptor (MET), rearranged during transfection (RET), human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma viral oncogene homolog (KRAS) as well as tumor protein 53 (TP53). National Comprehensive Cancer Network (NCCN) guidelines have suggested that it is necessary for patients with advanced or metastatic NSCLC to undergo genetic tests (6).
Given that more than 60% of the NSCLCs express EGFR, activating mutations in the EGFR gene occur in 32% of the NSCLC patients (7,8), and has become a meaningful target for anti-tumor therapy. Majority of the EGFR-activating mutations (~90%) primarily present exon 19 deletion (19 del; ~60%) or exon 21 point mutation L858R (~30%) (9). These genetic mutations promote the progression of NSCLC and affects the malignant proliferation, migration and survival of tumor cells by activating downstream signaling pathways. First-generation EGFR-tyrosine kinase inhibitor (TKI), gefitinib, was the first agent designed with a known molecular target to receive Food and Drug Administration (FDA) approval for treatment of lung cancer. However, its activity is limited to a subgroup of NSCLC cells with EGFR-sensitive mutations (10). In addition, the Iressa Pan-Asia Study (IPASS) clinical trial demonstrated that the first-generation EGFR-TKI can prolong progression-free survival in patients (9.5 vs. 6.3 months), but not overall survival (OS) (11).
On the other hand, osimertinib is an irreversible, third-generation EGFR-TKI that is highly selective for EGFR-activating mutations and the EGFR T790M mutation in advanced NSCLC with EGFR oncogene addiction (12). Osimertinib was approved in 2018 as a first-line treatment option for advanced EGFR-mutated NSCLC with or without the T790M mutation (13). A confirmatory, randomized, open label, international, phase 3 trial (AURA3) demonstrated that osimertinib had significantly greater efficacy compared to platinum therapy plus Pemetrexed in patients with T790M-positive mutations, including those with central nervous system (CNS) metastases who had NSCLC progression during first-line EGFR-TKI therapy (14).
Despite the documented efficacy of EGFR-TKIs, some patients develop resistance. The development of the T790M mutation is the main resistance mechanism associated with the first- and second-generation TKIs. The T790M mutation is located at EGFR exon 20, which is the site of action where EGFR inhibitors bind to ATP for their effects (15,16). Although osimertinib is effectively targeted against the T790M resistant mutation, the subsequent emergence of C797S still causes resistance. Which detected either in cis or trans with T790M. The EGFR C797S leads to disruption of the cysteine 797 binding site where osimertinib binds, and accounts for 40% of osimertinib resistance mechanisms (17,18).
Previous studies have shown that the allelic context that define C797S may predict responsiveness to alternative treatments (19). Patients with NSCLC harboring EGFR-sensitizing mutation, T790M, and in trans-C797S triple mutations, are resistant to third-generation EGFR TKIs, but sensitive to a combination of first and third generation TKIs. Previous data showed that if the allele gene is in cis, EGFR TKIs alone or in combination cannot suppress activity (20). Given the mutation by T790M in cis-C797S, all EGFR TKI alone do not work, and there is currently no better single treatment option after osimertinib resistance (20). Therefore, to achieve more effective clinical outcomes, there is need to understand the resistance mechanisms and enhance the development of effective treatment regimens.
Here, we present a patient who demonstrated EGFR mutations of 19 del, TP53 C124Lfs*25, T790M, and C797S mutation, occurrence of 96.79% (21) EGFR T790M reads against the same allele background (in cis) to EGFR C797S. The timeline of anti-cancer therapy is shown in Figure 1A. Unlike previous studies, who showed a optimize outcomes by the combination therapy of gefitinib and osimertinib.
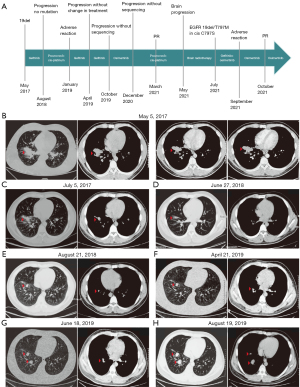
Case presentation
A 25-year-old man was admitted to the hospital complaints of fever, cough and chest distress which presented in May 2017 for 4 days. The patients did not have a history of smoking. Computed tomography (CT) scans from other hospitals showed a 28 mm × 29 mm mass in the inferior lobe of the right lung with metastases to the right lung, right pleura, right hilar and mediastinal lymph nodes (Figure 1B). CT-guided percutaneous pulmonary puncture confirmed LADCs with an EGFR exon 19 deletion mutation. Immunohistochemistry (IHC) staining showed positive results for creatine kinase (CK) and thyroid transcription factor (TIF). The patient was diagnosed with stage IIIA LADC with an EGFR exon 19 deletion mutation [cT3N2M0 according to tumor-node-metastasis (TNM) version 7].
Gefitinib (250 mg daily) was administered after the initial diagnosis as first-line treatment. The patient had significant positive response to the treatment and remained stable for 14 months as show in Figure 1C,1D. On August 21, 2018, patient reexamination revealed enlargement and progression of the primary lung lesion (Figure 1E), which was considered to have acquired resistance to gefitinib. However, second genetic tests of blood samples demonstrated that there was no gene mutation. Pemetrexed plus cis-platinum were administrated as second-line treatment options for three cycles between October and December 2018, and then the patient continued to use gefitinib owing to the adverse reaction of chemotherapy. Unfortunately, the disease progressed on April 21, 2019 (Figure 1F). Since the dressing was not changed, the CT reports on June 18, August 19, October 12, 2019, showed that the patient’s condition continued to progress (Figures 1G,1H,2A).
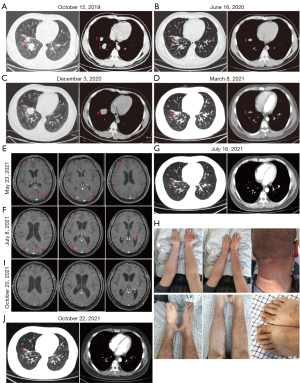
Without undergoing another genetic testing, the patient was given osimertinib as third-line treatment in October 2019. Partial remission of the primary lesion followed 14 months later (Figure 2B,2C).
In December 2020, the patient was examined for new symptoms of back pain. Chest and abdomen CT scan showed progression of the primary lesion (Figure 2C), splenomegaly, right supraclavicular lymph node, left submaxillary lymph node and right axillary lymph node enlargement; while emission computed tomography (ECT) scan demonstrated pulmonary bone disease; which together showed disease advancement. Pemetrexed plus cisplatin was administered on January 23, 2021, and follow-up CT showed shrinkage of the primary lesion (Figure 2D). On March 10 and April 18, 2021, the second and third cycles of Pemetrexed plus cisplatin palliative chemotherapy were administered. On May 23, 2021, the patient was presented to the hospital with headache and giddy. Cranial magnetic resonance imaging (MRI) enhancement demonstrated multiple metastatic brain tumors as shown in Figure 2E. The patient was put under palliative radiotherapy for brain cancer and intracranial pressure reduction treatment. On July 8, 2021, cranial MRI revealed no significant changes in brain metastases (Figure 2F). On July 16, 2021, chest CT results showed that the patient’s primary lesion had shrunk, but there were multiple metastases in both lungs (Figure 2G). On July 15, 2021, gene sequencing demonstrated original EGFR exon 19 deletion and acquisition of T790M in cis-C797S, and TP53 C124Lfs*25. The patient was first put on a combination treatment consisting of gefitinib (250 mg p.o. q.d.) and osimertinib (80 mg q.d.). Gefitinib was discontinued due to severe skin rash and paronychia by the end of September 2021 (Figure 2H), but osimertinib continued to be used. Bevacizumab (600 mg intravenously every 21 days) was also given to the patient during the radiotherapy and combination treatment.
On October 20, 2021, the MRI of the brain revealed disappearance of the brain metastases (Figure 2I). However, there was no significant change in lung metastases, and the primary lesion was slightly enlarged (Figure 2J). The patient passed away on June 12, 2022.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The pharmacological management of advanced NSCLC has revolutionized by the development of EGFR-TKIs. However, exposure of the NSCLC to EGFR-TKIs has led to inevitable development of resistance through EGFR dependent and independent mechanisms (18). The EGFR-dependent resistance mechanisms include acquired EGFR mutations such as EGFR T790M, C797S (22,23). Several EGFR-independent resistance mechanisms have also been reported.
In this study, we reported a LADC patient harboring EGFR mutation who had 19_del mutation to 19_del/T790M/in cis-C797S mutation after treatment with gefitinib (the first-generation EGFR-TKI) and osimertinib (third-generation EGFR-TKI). The patient initially underwent gefitinib and osimertinib combination treatment and there was disappearance of brain metastasis in three months.
Recent studies have demonstrated the development of C797S with respect to the other EGFR alleles which impact the efficacy of subsequent treatments (24). A previous in vitro demonstrated that a combination of first and third generation inhibitors would be effective if the C797S and T790M mutations were in trans but not in cis conformation (20). Besides, the first two cases showed that LADCs harboring EGFR 19Del/T790M/in trans-C797S were sensitive to the first- and third-generation EGFR TKI combination therapy. However, the two patients progressed rapidly, one and 3 months respectively, after the combination therapy (18,25). Another study showed that EGFR 19Del/T790M/in trans-C797S were sensitive to erlotinib and osimertinib combination therapy. However, the patient returned to the clinic and the disease deteriorated with the reappearance of EGFR T790M in cis-C797S (26).
Here, we report rare clinical case showing efficacy achieved by combination therapy of first and third EGFR-TKIs targeting concomitant EGFR T790M and C797S in cis conformation. Compared to previous studies that showed EGFR T790M in cis-C797S mutation was not responsive to gefitinib and osimertinib combination therapy, our patient is still receiving treatment and his brain lesions are being suppressed. Coupled with previous data, our findings suggest that, even if T790M and C797S are in cis, first-generation and third-generation EFGR-TKIs are effective in vivo. Once this allele is altered (cis into trans, or trans into cis), it may signal the emergence of another mechanism of resistance, which may trigger failure of the first generation and third generation treatment combination. Thus, there is need for more experimental and clinical studies to explore resistance mechanisms and exploration of target-specific drugs, which would provide more promising treatment options for treatment of patients with EGFR T790M and C797S.
Acknowledgments
Funding: The present study was supported by the College Student Innovation Training Program of Bengbu Medical College (Nos. Byycx21095 and Byycxz21069); 512 Talent Cultivation Plan of Bengbu Medical College (No. by51201319); Research and Innovation Team of Bengbu Medical College (No. BYKC201908); University Scientific Research Project of Education Department of Anhui Province (No. KJ2021A0714); and Provincial Education and Teaching Research Project (No. 2021jyxm0954).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1269/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021;127:3029-30. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- McKay JD, Hung RJ, Han Y, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet 2017;49:1126-32. [Crossref] [PubMed]
- Kohno T, Tsuta K, Tsuchihara K, et al. RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci 2013;104:1396-400. [Crossref] [PubMed]
- Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 2013;31:1097-104. [Crossref] [PubMed]
- Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res 2015;4:156-64. [PubMed]
- da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol 2011;6:49-69. [Crossref] [PubMed]
- Shah MP, Aredo JV, Padda SK, et al. EGFR exon 20 Insertion NSCLC and Response to Platinum-Based Chemotherapy. Clin Lung Cancer 2022;23:e148-53. [Crossref] [PubMed]
- Wang Q, Yang S, Wang K, et al. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol 2019;12:63. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Schmid S, Li JJN, Leighl NB. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer 2020;147:123-9. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Papadimitrakopoulou VA, Han JY, Ahn MJ, et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: Osimertinib versus platinum-Pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer 2020;126:373-80. [Crossref] [PubMed]
- Arulananda S, Do H, Musafer A, et al. Combination Osimertinib and Gefitinib in C797S and T790M EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1728-32. [Crossref] [PubMed]
- Zhao HY, Xi XX, Xin M, et al. Overcoming C797S mutation: The challenges and prospects of the fourth-generation EGFR-TKIs. Bioorg Chem 2022;128:106057. [Crossref] [PubMed]
- Niederst MJ, Hu H, Mulvey HE, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924-33. [Crossref] [PubMed]
- Genetic test results. 2021-07-23.
- Rangachari D, To C, Shpilsky JE, et al. EGFR-Mutated Lung Cancers Resistant to Osimertinib through EGFR C797S Respond to First-Generation Reversible EGFR Inhibitors but Eventually Acquire EGFR T790M/C797S in Preclinical Models and Clinical Samples. J Thorac Oncol 2019;14:1995-2002. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Duggirala KB, Lee Y, Lee K. Chronicles of EGFR Tyrosine Kinase Inhibitors: Targeting EGFR C797S Containing Triple Mutations. Biomol Ther (Seoul) 2022;30:19-27. [Crossref] [PubMed]
- Wang Z, Yang JJ, Huang J, et al. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J Thorac Oncol 2017;12:1723-7. [Crossref] [PubMed]
- Zhou Z, Zhao Y, Shen S, et al. Durable Clinical Response of Lung Adenocarcinoma Harboring EGFR 19Del/T790M/in trans-C797S to Combination Therapy of First- and Third-Generation EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 2019;14:e157-9. [Crossref] [PubMed]


