
Endovascular management and intravascular ultrasonography for spontaneous dissection of the internal carotid artery in acute stroke
Introduction
Arterial dissection is a disease in which blood components enter the vessel walls through the damaged intima of the artery (1,2). This leads to vessel wall dissection and stratification to form an intramural hematoma or protrusion outside the lumen contour to form a dissecting aneurysm, resulting in vascular stenosis, occlusion or rupture (3). Currently, the treatment options for extracranial arterial dissection (EAD) include medical conservative treatment, surgical treatment and endovascular interventional treatment. Medical conservative treatment is mainly anticoagulant assisted antiplatelet therapy; however, long-term applications of anticoagulant drugs increase the risk of intracerebral hemorrhage (4). Due to its high trauma and uncertain efficacy, surgical treatment is not the first choice for EAD (4). In recent years, advances in endovascular interventional therapy have improved its applications in EAD treatment (5). However, only a few case reports on spontaneous internal carotid artery (ICA) dissection treated with endovascular management assisted with intravascular ultrasonography (IVUS) are available. In this study, endovascular management assisted with IVUS was performed to help guide wire entry into the true lumen and prevent further dissection as well as cerebral infarction recurrence.
Case presentation
Clinical history and examination
A 59-year-old male patient was admitted with right limb weakness for 4 h, lethargy, inability to speak, crooked mouth, right limb muscle strength level 2 and positive Babinski sign of the right lower limb. Head computed tomography (CT) did not reveal bleeding. It was considered that the patient was very likely to have acute cerebral infarction and need interventional treatment immediately based on the CT report and our clinical experience. Considering the urgency of the situation, we did not perform any other non-invasive imaging modalities such as CT angiography (CTA) or diffusion magnetic resonance imaging (MRI) before performing angiography. Also, CTA was not as accurate as digital subtraction angiography (DSA) in detecting cerebral blood vessels, and CTA and MRI will cost more.
Routine intervention procedure
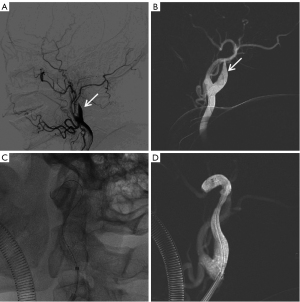
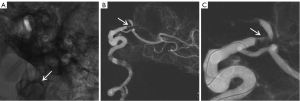
The patient was taken as an emergency, general anesthesia was induced, and endovascular intervention was performed via the right femoral approach. The operation began with intravenous heparin of 3,000 U and was followed by another 1,000 U heparin every hour to maintain active coagulation time of 250–300 s. Angiography (cordis angiographic catheter, 5-F, 451-514H0) revealed occlusion of the C1 segment of the left ICA (LICA) (Figure 1A,1B). Under the support of guiding catheter and a microcatheter (EV3 Rebar-18), after repeated adjustments, the guide wire (Stryker Synchro2) and another guide wire (ASAHI Fielder XT-A) could not pass through the occlusion section of the LICA and reach the distal vessel (Figure 1C). Angiography revealed a suspicious LICA dissection (Figure 1D).
The IVUS technique
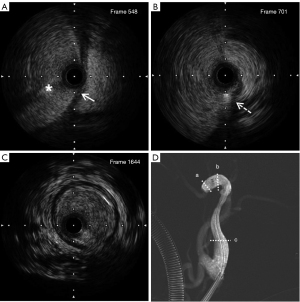
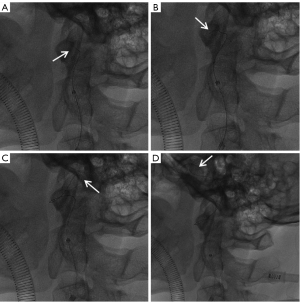
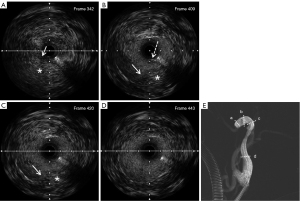
An intravascular ultrasound catheter (Boston Scientific, 3.0-F OptiCross) was inserted into the segment of the suspicious false lumen, and the IVUS movie images were obtained using a electric pull-back system. It was confirmed that the guide wire was located in the false lumen (Figure 2A). During ultrasound catheter withdrawal, the dissection break was detected and recorded (Figure 2B-2D). The IVUS image showed a dissection with an intimal flap visible and the true cavity narrowed to 70% of its normal diameter. Using the location identified by IVUS, the guide wire was adjusted to smoothly re-enter the true cavity (Figure 4). The IVUS catheter was inserted again, which confirmed that the guide wire was located in the true lumen (Figure 5). There was a massive hematoma in the interlayer (Figure 5A).
Successful operation
Blood flow in the C1–C4 segments of the LICA was improved after balloon expansion (Hoper, 2.0 mm × 15 mm) (Figure 3A). With sented microcatheter, the thrombectomy bracket (Nico, 5 to 30 mm) was released in the ophthalmic artery segment, anterior cerebral artery and middle cerebral artery after which a large number of dark red thrombus were removed. Re-examination showed that the LICA, left anterior cerebral artery and left middle cerebral artery M1 segments were unobstructed, while the left middle cerebral artery M2 segment was occluded (Figure 3B). Due to tortuous blood vessels in the patient’s neck, the maximum length of the intermediate catheter and micro-catheter could only reach the proximal end of M2, thus, the M2 segment could not be opened further and the operation was ended. A total of 6,000 U heparin was used during the operation and aspirin 100 mg once daily (q.d.) and clopidogrel 75 mg q.d.
On the 4th day after interventional therapy, the patient underwent decompressive craniotomy due to massive cerebral infarction and consciousness disturbance. Postoperative the patient received brain protection, intracranial pressure control, rehabilitation exercise and other treatments. After 20 days of hospitalization, the patient was discharged from the hospital in fair condition, with right upper limb muscle strength level 1, right lower limb muscle strength level 2, and left limb muscle strength was normal. Four months later, the patient had grade 1 muscle strength in the right upper limb and grade 4 muscle strength in the right lower limb. The patient underwent repair of left frontotemporal top skull defect, and the operation was successful. The follow-up CT images was in Figure S1.
All the study procedures were in accordance with the ethical standards of the Chongqing General Hospital research committee(s) and the Declaration of Helsinki (as revised in 2013). A written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
EAD is associated with carotid artery occlusion and the formation as well as shedding of unstable thrombotic plaques in the dissection aneurysm (1,2). Clinically, it is one of the important causes of ischemic stroke in young and middle-aged adults. About 15–25% of stroke in patients younger than 45 years is attributed to carotid EAD (3). Hypertension is a common risk factor for EAD. In addition, a history of hyperlipidemia, connective tissue disease, myofibrous dysplasia, Marfan syndrome, trauma, and iatrogenic injury are risk factors for EAD (2,6). The symptoms of EAD vary, and most patients with mild lesions have no obvious discomforts or mild symptoms, which are only accidentally found during physical examinations. Patients with more severe lesions often exhibit ischemic stroke and other non-cerebral ischemia symptoms such as headache, neck pain, Horner syndrome, and cranial nerve paralysis, among others (2,6).
Various imaging methods have suggestive effects on EAD. For instance, MRI T1-weighted imaging (T1WI) can show arterial wall hematoma, T2WI can show intimal flap, cranial magnetic resonance angiography (MRA) and CTA can show arterial occlusion, rat tail sign, double lumen sign, and intimal valve. Clinically, DSA imaging is the gold standard for EAD diagnosis. Dissections with diameters of less than 2 mm can be found by DSA examination. The complex peripheral vascular relationship can be revealed by three-dimensional (3D)-DSA, which still plays an irreplaceable role in EAD diagnosis and typing (7,8). It is challenging for the guide wire to pass through the dissection, especially for occlusion lesions (9). In percutaneous coronary intervention, intravascular ultrasound has been able to identify the different layers of the artery wall that have been peeled off, such as the intima, the media, and the outer membrane, and ultimately the peeling intima flap (10).
In this case, we reported spontaneous ICA dissection treated with IVUS-guided. The benefits of IVUS-guided carotid artery stenting (CAS) for intracranial atherosclerosis (ICAS) were evaluated and confirmed through multiple studies with a debate on whether the routine use of IVUS during CAS is recommended (11,12). Nevertheless, IVUS-guided for intracranial dissection (ICD) was rarely investigated with few case reports on vertebral artery dissection (13) and ICA dissection (14). In addition, the highlight of our report was that we reported a case in which IVUS guided the micro-guidewire into the true lumen of the ICA dissection, but previously reports were just using IVUS as a guide for stent placement and post-stenting confirmation.
IVUS confirmed that the microguide wire had been inserted into the true cavity. The treatment of intravascular dissection is challenging because it is difficult to determine whether a putative true cavity is an actual cavity. Therefore, IVUS has a key role to play in overcoming this challenge. Visualization of the dissected luminal environment by conventional angiography was not achieved but was achieved with IVUS. In this case, before the use of IVUS, the microguidewire did not pass through the occlusive vessel, but entered the false lumen through the dissection break. Under the guidance of IVUS, the microguidewire passed through the dissection break into the true lumen at the distal end of the occlusive vessel and the vessel was finally opened. The IVUS was critical to the success of the operation.
Once it had been confirmed that the distal end of the microcatheter was in the true lumen, appropriate vascular reconstructions were performed. In this case, the patient with acute vascular occlusion due to dissection was reconstructed by balloon dilatation rather than stent implantation. Since this was a tandem lesion, that is, there was proximal carotid artery dissection with occluded and distal carotid artery thrombosis in the ocular segment, it was not viable to place the stent at the proximal carotid artery.
Conclusions
Treatment of spontaneous ICA dissection under IVUS guidance was established to be safe and feasible. The intravascular environment can be clearly shown using an IVUS, and the technique helps to hone the cavity. Clinical experience with this technology is necessary and mandatory, and smaller diameter and better traceable devices are essential for further introduction of IVUS into the field of endovascular neurosurgery.
Acknowledgments
The authors appreciate Dewei Zou and Longwei Zeng (Department of Neurosurgery, Chongqing General Hospital, Chongqing, China) for performing this surgery together.
Funding: This study was supported by grants from the Chongqing Natural Science Foundation project (No. CSTB2022NSCQ-MSX1563) and the Basic Research and Frontier Exploration Project of Yuzhong District, Chongqing (No. 20210167).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1337/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the study procedures were in accordance with the ethical standards of the Chongqing General Hospital research committee(s) and the Declaration of Helsinki (as revised in 2013). A written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Todua F, Gachechiladze D. Dissection of Extracranial Arteries. In: Todua F, Gachechiladze D. editors. Noninvasive Radiologic Diagnosis of Extracranial Vascular Pathologies. Cham: Springer, 2018.
- Kwon JY, Kim NY, Suh DC, Kang DW, Kwon SU, Kim JS. Intracranial and extracranial arterial dissection presenting with ischemic stroke: Lesion location and stroke mechanism. J Neurol Sci 2015;358:371-6. [Crossref] [PubMed]
- Luo Y, Guo ZN, Niu PP, Liu Y, Zhou HW, Jin H, Yang Y. 3D T1-weighted black blood sequence at 3.0 Tesla for the diagnosis of cervical artery dissection. Stroke Vasc Neurol 2016;1:140-6. [Crossref] [PubMed]
- Chowdhury MM, Sabbagh CN, Jackson D, Coughlin PA, Ghosh J. Antithrombotic treatment for acute extracranial carotid artery dissections: a meta-analysis. Eur J Vasc Endovasc Surg 2015;50:148-56. [Crossref] [PubMed]
- Alberts MJ, Shang T, Magadan A. Endovascular Therapy for Acute Ischemic Stroke: Dawn of a New Era. JAMA Neurol 2015;72:1101-3. [Crossref] [PubMed]
- Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 2015;14:640-54. [Crossref] [PubMed]
- Kobayashi H, Morishita T, Ogata T, Matsumoto J, Okawa M, Higashi T, Inoue T. Extracranial and intracranial vertebral artery dissections: A comparison of clinical findings. J Neurol Sci 2016;362:244-50. [Crossref] [PubMed]
- Chen H, Hong H, Xing S, Liu G, Zhang A, Tan S, Zhang J, Zeng J. Intracranial versus extracranial artery dissection cases presenting with ischemic stroke. J Stroke Cerebrovasc Dis 2015;24:852-9. [Crossref] [PubMed]
- Daoulah A, Al Qahtani A, Mazen Malak M, Al Ghamdi S. Role of IVUS in Assessing Spontaneous Coronary Dissection: a Case Report. J Tehran Heart Cent 2012;7:78-81. [PubMed]
- Kwon TG, Cho YJ, Bae JH. Physical Principles and Equipment: IVUS. In: Hong MK. editor. Coronary Imaging and Physiology. Singapore: Springer, 2018.
- Clark DJ, Lessio S, O'Donoghue M, Schainfeld R, Rosenfield K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv 2004;63:355-62. [Crossref] [PubMed]
- Chiocchi M, Morosetti D, Chiaravalloti A, Loreni G, Gandini R, Simonetti G. Intravascular ultrasound assisted carotid artery stenting: randomized controlled trial. Preliminary results on 60 patients. J Cardiovasc Med (Hagerstown) 2019;20:248-52. [Crossref] [PubMed]
- Seung WB. Stent-Assisted Angioplasty of Spontaneous Bilateral Extracranial Vertebral Dissections under Intravascular Ultrasound Guidance. Case Rep Neurol 2018;10:314-21. [Crossref] [PubMed]
- Zlatancheva G, Vassilev D, Karamfilov K, Petrova J, Zlatareva D, Gil RJ. Intravascular ultrasound-guided primary stenting of spontaneous carotid artery dissection. Kardiol Pol 2022;80:702-4. [Crossref] [PubMed]


