
CD19/CD22 chimeric antigen receptor (CAR) T-cell cocktail therapy following autologous transplantation in patients with relapsed/refractory diffuse large B-cell lymphoma on 18F-FDG PET/CT: a case description
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has achieved great clinical success in treating patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) (1-3). High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 gene rearrangements, defined as double-hit lymphoma (DHL), accounts for approximately 2–10% of newly diagnosed DLBCL cases (4). Most studies have shown that DHL has a poor prognosis with standard chemoimmunotherapy, and it does not derive apparent benefit from consolidative autologous stem-cell transplantation (ASCT) (5-7). The ZUMA-7 trial shows significantly improved efficacy of the CAR-T cell therapy axicabtagene ciloleucel in R/R DLBCL patients (8). Although ASCT plays a potentially curative role in treating R/R DLBCL, the prognosis of R/R DLBCL patients remains poor, and more novel strategies are needed compared with standard ASCT alone (9). Preliminary results have shown that donor-derived allogeneic CAR-T cells can cause regressions of B cell malignancies refractory to other therapies without causing graft-versus-host disease (GVHD) (10,11).
In the present work, we described an R/R DLBCL patient who received ASCT and CD19/22 CAR-T cell cocktail therapy and achieved excellent disease response without the residual lesion. He remained asymptomatic for 38 months of follow-up.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
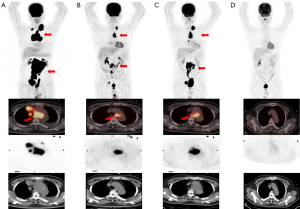
In May 2019, a 50-year-old male was diagnosed with DLBCL, germinal center B-cell (GCB) subtype, MYC and BCL6 translocation (DHL). Baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) was then performed for lymphoma staging. PET/CT (Figure 1A) showed lymphadenopathy in bilateral cervical, mediastinum, retroperitoneum, abdominal, and pelvic cavities, some of which fused into a mass (11.3 cm × 8.5 cm), with various degrees of abnormal FDG uptake and maximum standardized uptake value (SUVmax) ranging from 5.4 to 17.3. He was treated with four cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). Interim 18F-FDG PET/CT (Figure 1B) showed a significant decrease in lymph node volume (4.3 cm × 2.1 cm) but similar 18F-FDG uptake (SUVmax 16.8) and a score of 5 by Deauville criteria. Therefore, two cycles of R-Gemox (ituximab, gemcitabine, and oxaliplatin) chemotherapy were given. Unfortunately, PET/CT showed increased lesion volume with radioactive uptake, indicating disease progression (SUVmax: 14.98; 7.6 cm × 4.3 cm; Figure 1C). The patient received CD19/22 CAR-T cells at a dose of 1.0×107/kg on Dec 31, 2019. Prior to CAR-T cell infusion, the patient received decitabine (50 mg/m2) in combination with FC regimen (fludarabine 50 mg/m2 and cyclophosphamide 200 mg/m2) as lymphodepletion chemotherapy for three consecutive days. The CAR transfection efficiency was 34.4%, with 86.95% CAR-T+CD4+ cells and 12.37% CAR-T+CD8+ cells. From day 3 to day 7, the patient had a fever with a highest temperature of 39.5 ℃. On day 8, the peak of serum IL-6 reached 2,870.5 pg/mL, leading to a diagnosis of grade 3 cytokine release syndrome (CRS). Anti-infective treatment was administered based on the positive blood culture results, which suggested catheterized non-fermenting corynebacterium. Subsequently, he was administered radiotherapy [volumetric modulated arc therapy (VMAT) nasopharynx gross tumor volume (GTVnx): 40 Gy/20 f] and bendamustine-rituximab (BR) chemotherapy, and the PET/CT indicated a complete metabolic response with no abnormal FDG uptake (Figure 1D).
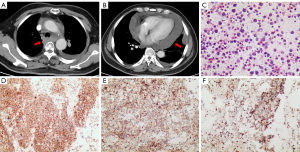
At 18 months after the primary diagnosis, the patient suddenly presented dyspnea and wheezing. Therefore, he underwent a CT scan, revealing mediastinal, hilar enlarged lymph nodes, as well as large pericardial and pleural effusions (Figure 2). Biopsy from the pericardial effusion was suggestive of lymphoma involvement. Subsequently, on Jan 4, 2021, the patient underwent allogeneic hematopoietic stem-cell transplantation (allo-HSCT). Unfortunately, 3 months after allo-HSCT, a biopsy confirmed that the patient relapsed with fever and pericardial and pericardial effusions.
Then, he received a second CD22/19 CAR-T cell therapy on June 30, 2021. The patient received lymphodepletion chemotherapy with fludarabine (50 mg/m2, days −5 to −3) before CAR-T cell therapy. A total of 1.0×107/kg CAR-T cells were infused in the patient for three consecutive days (10%, 30%, and 60% of total dose). The transduction efficiency of the CAR-T cells was 38.13%, including 84.53% of CAR-T+CD4+ cells and 14.27% of CAR-T+CD8+ cells. Chest tightness occurred after reinfusion, which was considered CAR-T-related capillary leak syndrome, and dexamethasone (DEX) 10 mg once daily for 1 day was given to suppress the inflammatory response. No CAR-T cell-associated neurological toxicities were observed after the infusion.
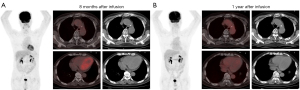
Peripheral blood lymphocytes were obtained from the patient via leukapheresis. T cells were isolated using anti-CD3 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), anti-CD3/CD28 monoclonal antibodies (Miltenyi Biotec, Bergisch Gladbach, Germany) were used to stimulate cell proliferation. T cells were subsequently transduced with a recombinant lentiviral vector and cultured in AIM-V media (Gibco, NY, USA), supplemented with 10% autologous human serum, 100 IU/mL IL-2, 5 ng/mL IL-7, and 5 ng/mL IL-15 for 12–14 days before infusion. The CAR-T cells were provided by Shanghai Unicar-Therapy Bio-Medicine Technology Co. (Shanghai, China). At 8 months after infusion, PET/CT showed no abnormal radioactive uptake by the lymphoma and a small amount of pericardial effusion (Figure 3A). PET/CT images 1 year after CAR-T cell infusion showed no residual lesions with a Deauville score of 1 (Figure 3B). He remained asymptomatic for 38 months of follow-up.
Discussion
R/R DLBCL patients who do not respond to first-line therapy are unlikely to respond to high-dose therapy with ASCT. In this case, a change to third-line therapy is required, sometimes in the absence of progressive disease.
CAR-T cell therapy has shown unexpected results in patients with R/R DLBCL. Therefore, National Comprehensive Cancer Network guidelines recommend it as a third-line treatment for patients with R/R aggressive B-cell non-Hodgkin lymphoma (B-NHL). Furthermore, the results of CAR-T cell immunotherapy following ASCT in patients with R/R DLBCL show a significant improvement in prognosis. This finding encourages further trials to explore whether continuous CAR-T cell-targeted therapy after high-dose therapy and autologous stem cell transplant improves the outcomes of the suboptimal group of patients with R/R aggressive B-NHL who have failed to salvage chemotherapy (12).
Several studies have shown that interventions with conditional modification and maintenance therapy cannot achieve long-term disease-free survival, partially due to positive pre-transplant minimal residual disease after salvage chemotherapy. The 2-year progression-free survival (PFS) of patients with PET/CT positivity after salvage chemotherapy ranges from 30% to 50% (13-15). Numerous studies have identified PET/CT scans as a main prognostic factor in patients with R/R B-cell lymphoma, overshadowing International Prognostic Index (IPI) scores, Eastern Cooperative Oncology Group (ECOG) scores, and lactate dehydrogenase (LDH) levels for both PFS and overall survival (OS) (14). Zhang et al. (14) have assessed the prognostic value of pre-transplant 18F-FDG PET/CT in 46 DLBCL patients following salvage chemotherapy. Compared with PET-negative patients, the positive group has a high risk of ASCT failure.
18F-FDG PET/CT serial imaging is essential in assessing disease extent, staging, monitoring treatment response, and detecting recurrence. Patients with a positive FDG PET/CT after chemotherapy had a worse prognosis. Our results suggested that patients with a positive interim PET could be candidates for novel therapeutic strategies.
Conclusions
The survival time of this patient with R/R DLBCL after CAR-T cell immunotherapy and allo-HSCT was prolonged. Our findings provided a promising novel treatment option for R/R DLBCL patients 18F-FDG PET/CT imaging remained an excellent imaging modality to assess the extent of disease and response to treatment in lymphoma. In our case, serial 18F-FDG PET/CT helped monitor treatment response.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1209/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31-42. [Crossref] [PubMed]
- Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med 2017;377:2545-54. [Crossref] [PubMed]
- Breen WG, Hathcock MA, Young JR, Kowalchuk RO, Bansal R, Khurana A, Bennani NN, Paludo J, Villasboas Bisneto JC, Wang Y, Ansell SM, Peterson JL, Johnston PB, Lester SC, Lin Y. Metabolic characteristics and prognostic differentiation of aggressive lymphoma using one-month post-CAR-T FDG PET/CT. J Hematol Oncol 2022;15:36. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Chen AI, Leonard JT, Okada CY, Gay ND, Chansky K, Fan G, Dunlap JB, Raess PW, Braziel RM, Stentz A, Maziarz RT. Outcomes of DA-EPOCH-R induction plus autologous transplant consolidation for double hit lymphoma. Leuk Lymphoma 2018;59:1884-9. [Crossref] [PubMed]
- Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol 2015;16:e555-67. [Crossref] [PubMed]
- Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3452-9. [Crossref] [PubMed]
- Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med 2022;386:640-54. [Crossref] [PubMed]
- Herrera AF, Mei M, Low L, Kim HT, Griffin GK, Song JY, et al. Relapsed or Refractory Double-Expressor and Double-Hit Lymphomas Have Inferior Progression-Free Survival After Autologous Stem-Cell Transplantation. J Clin Oncol 2017;35:24-31. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013;122:4129-39. [Crossref] [PubMed]
- Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol 2016;34:1112-21. [Crossref] [PubMed]
- Cao Y, Xiao Y, Wang N, Wang G, Huang L, Hong Z, et al. CD19/CD22 Chimeric Antigen Receptor T Cell Cocktail Therapy following Autologous Transplantation in Patients with Relapsed/Refractory Aggressive B Cell Lymphomas. Transplant Cell Ther 2021;27:910.e1-910.e11. [Crossref] [PubMed]
- Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, Hilden P, Devlin SM, Zelenetz AD, Moskowitz CH. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood 2015;125:2579-81. [Crossref] [PubMed]
- Zhang XY, Song L, Wang PJ, Wang L, Li Y, Wang YY, Shi CL. Prognostic Value of Pre-Autologous Stem Cell Transplantation PET/CT in Diffuse Large B-Cell Lymphoma: The Deauville Score Is Prognostically Superior to ΔSUVmax. Acta Haematol 2020;143:124-30. [Crossref] [PubMed]
- Armand P, Welch S, Kim HT, LaCasce AS, Jacobsen ED, Davids MS, Jacobson C, Fisher DC, Brown JR, Coughlin E, Freedman AS, Chen YB. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br J Haematol 2013;160:608-17. [Crossref] [PubMed]


