Magnetic resonance enterography findings of a gastrocolic fistula in Crohn’s disease
Crohn’s disease (CD) is characterized by patches of inflammation, which may affect the whole gastro-intestinal tract. Internal fistulization is a common complication of CD due to the transmural nature of inflammation. However, gastrocolic fistulas are rare in CD. We present the magnetic resonance enterography (MRE) findings of a gastrocolic fistula in a patient with longstanding CD with clinical and pathologic correlation.
Case presentation
A 29-year-old woman with perianal fistulizing CD visited our hospital for left-sided upper abdominal pain starting about 30 minutes after the meals, bloating, diarrhea, anorexia, and weight loss. Laboratory results showed a slight anemia and a moderate increased C-reactive protein. A colonoscopy was performed and showed a stenosis at the splenic flexure with suspicion of an internal fistula in the inflamed area. Drug therapy was intensified, without relief of symptoms. A new colonoscopy was performed with dilation of the stenosis, also with insufficient result.
MRE showed abnormal wall thickening and increased bowel wall enhancement involving the splenic flexure of the colon along with extensive perimural inflammation. This resulted in a stricture extending over 10 centimeters. The dynamic contrast series showed a tubular slightly hyperenhancing structure connecting the splenic flexure with the stomach, indicative for a gastrocolic fistula (Figure 1). The stomach, remaining colon and small bowel (SB) including the terminal ileum, showed no signs of CD involvement.
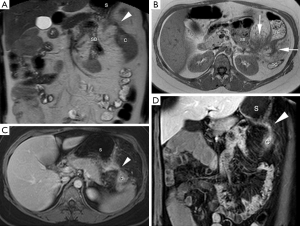
Definitive treatment was established by segment resection of the splenic flexure with stapling of the gastrocolic fistula (Figure 1). The postoperative course was unremarkable and patient recovered uneventfully.
Gross pathological examination of the surgical specimen confirmed the presence of fistulous disease. A deep penetrating inflammatory process originated from the colonic mucosa, extended through the colonic wall and attached to the stomach. In this inflammatory tract, gastric mucosa was found represented by glands formed by parietal and chief cells of fundic mucosa.
Discussion
CD is a chronic inflammatory disorder characterized by discontinuous inflammatory lesions that may affect the whole gastro-intestinal tract, albeit most commonly the small and large bowel. Most patients present with abdominal pain and diarrhea, and typically follow a relapsing and remitting course. According to the Montreal classification of inflammatory bowel disease, three patterns of CD are distinguished; non-stricturing/non-penetrating disease, stricturing (stenotic or obstructive) disease, and penetrating (fistulous, with inflammatory masses and/or abscesses) disease (1). Although this classification suggests that patients have a tendency to progress toward either structuring or penetrating disease, both disease patterns can coexist as in our case (2). MRE can help to establish the presence of these different CD phenotypes.
Transmural inflammation is typical for CD and likely predisposes to the formation of sinus tracts and fistulas. About 34–50% of CD patients are affected by one or more fistulas and this number increases along with disease duration (3). Most fistulas occur in the perianal region (4). These and other fistulas that penetrate the cutaneous surface are called external fistulas. Conversely, fistulas that connect with another internal organ, retroperitoneal space, thorax or blood vessels are referred to as internal fistulas (4).
Although fistulas are frequently seen originating from CD, the formation of a gastrocolic fistula is rare. The first case was described in 1937 by Bargen et al. from the Mayo clinic (5) and since then only few cases are reported in the literature (5-9). To best of our knowledge, our case is the first documentation of a gastrocolic fistula by MRE in a patient with CD. Gastrocolic fistulas in CD mostly arise from the affected transverse colon (TC) and extend by the gastrocolic ligament to involve the greater curvature of the stomach secondarily, usually without gastric involvement (6). Hence, the term colonogastric fistula is advocated, as it describes this entity more appropriately, but not widely accepted.
Although gastrocolic fistulas in CD are uncommon, various other diseases may be the cause. Among these are previous surgeries, carcinomas or carcinoid of the stomach or the TC, trauma, intra-abdominal abscesses, pancreatic carcinoma, and diverticulitis.
The classical triad of symptoms for gastrocolic fistulas in CD is diarrhea, weight loss, and fecal halitosis or fecal vomiting, albeit this is only present in about 30% of patients. Most patients have diarrhea, abdominal pain, and weight loss, but these symptoms fail to distinguish a gastrocolic fistula from an uncomplicated exacerbation of CD (6).
Computed tomography enterography (CTE) and MRE are the imaging modalities of choice for overall assessment of CD because of exquisite image quality, rapid acquisition time, and lack of need for bowel preparation (10,11). The fundamental role of cross-sectional imaging techniques in CD is recently exemplified by a retrospective study including 56 patients with established CD. In half of patients diagnosed with penetrating disease, this complication was clinically occult. Subsequently, therapy was altered in 79% of these patients (12). MRE is highly accurate in detecting internal fistulas in CD, with sensitivities ranging from 71–100% and specificities from 92–100% (13,14). MRE is superior to CTE in detecting internal and perianal fistulas (15,16), has the major advantage of lacking ionizing radiation (11,15-17) and has better contrast resolution (10,18). Therefore, MRE is the imaging modality of choice in CD, especially in young patients and for those requiring repetitive imaging (17,18).
Findings of CD on MRE are thickened bowel wall (>3 mm), increased wall enhancement, bowel wall edema, ulceration, perimural inflammation, and lymph node enhancement. Specific MR features for CD are creeping fat (increased mesenteric fat), skip lesions and fistulas, that can be identified on both morphologic T2 sequences and dynamic contrast-enhanced series (8,15). In our case, the gastrocolic fistula was nicely depicted both on T2-weighted sequence and dynamic contrast-enhanced series (Figure 1). Increased enhancement of the fistulous tract correlated well with active ongoing CD by histopathology.
Intensifying medical therapy is an accepted strategy for gastrocolic fistulas in CD with significant improvement of symptoms and even radiological resolution in some cases (19). When symptoms persist, resection of the diseased colonic segment with closure of the gastric defect is required (6).
In conclusion, we present the first MRE findings of a gastrocolic fistula in CD. MRE is the imaging modality of choice for overall assessment of CD, including the detection of internal fistulas.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749-53. [Crossref] [PubMed]
- Gupta N, Bostrom AG, Kirschner BS, Ferry GD, Gold BD, Cohen SA, Winter HS, Baldassano RN, Abramson O, Smith T, Heyman MB. Incidence of stricturing and penetrating complications of Crohn's disease diagnosed in pediatric patients. Inflamm Bowel Dis 2010;16:638-44. [Crossref] [PubMed]
- Nielsen OH, Rogler G, Hahnloser D, Thomsen OØ. Diagnosis and management of fistulizing Crohn's disease. Nat Clin Pract Gastroenterol Hepatol 2009;6:92-106. [Crossref] [PubMed]
- Herrmann KA, Michaely HJ, Zech CJ, Seiderer J, Reiser MF, Schoenberg SO. Internal fistulas in Crohn disease: magnetic resonance enteroclysis. Abdom Imaging 2006;31:675-87. [Crossref] [PubMed]
- Logio T, Chaiken B, Roth J, Newman E, Siegel T. The management of Crohn's colitis with colonogastric fistula. Report of a case. Dis Colon Rectum 1987;30:699-704. [Crossref] [PubMed]
- Khanna MP, Gordon PH. Gastrocolic fistulization in Crohn's disease: a case report and a review of the literature. Can J Surg 2000;43:53-6. [PubMed]
- Flueckiger F, Kullnig P, Melzer G, Posch E. Colobronchial and gastrocolic fistulas: rare complication of Crohn's disease. Gastrointest Radiol 1990;15:288-90. [Crossref] [PubMed]
- Greenstein AJ, Present DH, Sachar DB, Slater G, Heimann T, Lachman P, Aufses AH Jr. Gastric fistulas in Crohn's disease. Report of cases. Dis Colon Rectum 1989;32:888-92. [Crossref] [PubMed]
- Gidvani V, Mizell L. Gastrocolic fistula in pediatric Crohn's disease. J Pediatr Gastroenterol Nutr 1997;25:347-9. [Crossref] [PubMed]
- Towbin AJ, Sullivan J, Denson LA, Wallihan DB, Podberesky DJ. CT and MR enterography in children and adolescents with inflammatory bowel disease. Radiographics 2013;33:1843-60. [Crossref] [PubMed]
- Masselli G, Gualdi G. CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom Imaging 2013;38:249-59. [Crossref] [PubMed]
- Booya F, Akram S, Fletcher JG, Huprich JE, Johnson CD, Fidler JL, Barlow JM, Solem CA, Sandborn WJ, Loftus EV Jr. CT enterography and fistulizing Crohn's disease: clinical benefit and radiographic findings. Abdom Imaging 2009;34:467-75. [Crossref] [PubMed]
- Maccioni F, Bruni A, Viscido A, Colaiacomo MC, Cocco A, Montesani C, Caprilli R, Marini M. MR imaging in patients with Crohn disease: value of T2- versus T1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology 2006;238:517-30. [Crossref] [PubMed]
- Martínez MJ, Ripollés T, Paredes JM, Blanc E, Martí-Bonmatí L. Assessment of the extension and the inflammatory activity in Crohn's disease: comparison of ultrasound and MRI. Abdom Imaging 2009;34:141-8. [Crossref] [PubMed]
- Ippolito D, Invernizzi F, Galimberti S, Panelli MR, Sironi S. MR enterography with polyethylene glycol as oral contrast medium in the follow-up of patients with Crohn disease: comparison with CT enterography. Abdom Imaging 2010;35:563-70. [Crossref] [PubMed]
- Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, Fries W, Balzarini L, Montorsi M, Malesci A, Danese S. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn's disease. Inflamm Bowel Dis 2011;17:1073-80. [Crossref] [PubMed]
- Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C, Peyrin-Biroulet L, Rimola J, Rogler G, van Assche G, Ardizzone S, Ba-Ssalamah A, Bali MA, Bellini D, Biancone L, Castiglione F, Ehehalt R, Grassi R, Kucharzik T, Maccioni F, Maconi G, Magro F, Martín-Comín J, Morana G, Pendsé D, Sebastian S, Signore A, Tolan D, Tielbeek JA, Weishaupt D, Wiarda B, Laghi A. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 2013;7:556-85. [Crossref] [PubMed]
- Grand DJ, Guglielmo FF, Al-Hawary MM. MR enterography in Crohn's disease: current consensus on optimal imaging technique and future advances from the SAR Crohn's disease-focused panel. Abdom Imaging 2015;40:953-64. [Crossref] [PubMed]
- Levy C, Tremaine WJ. Management of internal fistulas in Crohn's disease. Inflamm Bowel Dis 2002;8:106-11. [Crossref] [PubMed]


