
Morphology of hypertrophied basal septum contributes to left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy: a retrospective case-control study
Introduction
Hypertrophic cardiomyopathy (HCM) is an inherited cardiac disease characterized by unexplained cardiac hypertrophy (1,2), with a prevalence ranging from 0.2% to 0.5% in young adults in the United States (3). Left ventricular outflow tract obstruction (LVOTO), also known as hypertrophic obstructive cardiomyopathy (HOCM), is a major hallmark of HCM and occurs in approximately two-thirds of HCM patients (4). LVOTO is associated with adverse outcomes in patients with HCM, and clinical symptoms include dyspnea, chest pain, palpitations, and syncope (1).
The mechanism of LVOTO in patients with HCM was more complex than it initially seemed. The integration of various factors may responsible for the occurrence of LVOTO: narrowing of the left ventricular outflow tract (LVOT) resulting from the hypertrophied interventricular septum (IVS), abnormalities of mitral valve (MV) apparatus, and abnormal submitral apparatus [such as anomalous MV leaflets and papillary muscle (PM), abnormal muscle bundles (MBs)] give rise to systolic anterior motion (SAM) and LVOTO (5-8). Septal hypertrophy is one of the major characteristics of HCM, and the thickness ≥30 mm is associated with sudden cardiac death (9). The main reasons for LVOTO were attributed to septal hypertrophy and SAM (10). Septal hypertrophy may narrow the LVOT, which is sufficient to accelerate blood flow, so that the subsequent decrease in pressure could generate a drag force to pull the MV anteriorly towards the septum (7).
Echocardiography is the first-line imaging modality used to evaluate the cardiac function and structure. With routine echocardiography, we found that some patients with basal septal hypertrophy did not present with LVOTO. Meanwhile, we encountered a subset of HCM cases with the same degree of basal septal hypertrophy but with varied occurrence of LVOTO. Through preliminary observation, we found that there were differences among these patients in the morphology of the hypertrophied basal septum (BS) and its surrounding structures. However, the existing literature on the morphology of the BS, its relationship with the surrounding structures, and their role in LVOTO in HCM patients is limited. Previous studies about the morphology of the BS mainly focused on the thickness or qualitative analysis (11,12). In this study, we aimed to quantify the morphology of the BS in patients with HCM, and we created a new method for measuring the BS. We aimed to (I) explore the differences in hypertrophied basal septal morphology between HCM patients and healthy individuals, and (II) explore the differences in the morphology of hypertrophied BS and surrounding structures between obstructive HCM and nonobstructive HCM patients, thus identifying their role in LVOTO. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1034/rc).
Methods
Study population
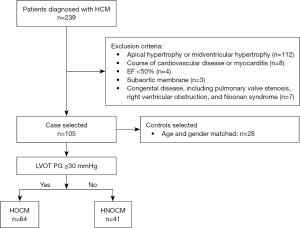
We consecutively enrolled 239 patients diagnosed with HCM between January 2019 and December 2019 at Fuwai Hospital. The exclusion criteria included: (I) apical hypertrophy or midventricular hypertrophy (n=112); (II) course of cardiovascular disease or myocarditis (n=8); (III) ejection fraction (EF) <50% (n=4); (IV) subaortic membrane (n=3); and (V) congenital disease, including pulmonary valve stenosis, right ventricular obstruction, and Noonan syndrome (n=7). According to the preliminary experiment results and sample size calculation formula (13): n=2 [Zα + Z (1 – β)]2 × SD2/d2, we got a minimum sample size of 11 in each group using a two-sided significance level α of 5% and a power of 0.8. Echocardiographic digital database of 105 patients was retrospectively analyzed (Figure 1). The controls comprised 28 age- and gender-matched individuals with no history of hypertension or cardiovascular disease, who underwent transthoracic echocardiography (TTE) for chest pain or dyspnea, and were found to have no echocardiographic abnormalities. According to the guidelines for HCM (3,14), a diagnosis of HCM was made when unexplained hypertrophy of any myocardial segment (wall thickness ≥15 or ≥13 mm in patients with a family history) was shown on two-dimensional echocardiography or cardiovascular magnetic resonance; patients refractory to pharmacologic therapy and with an LVOT gradient ≥50 mmHg at rest or during provocation should be referred for surgery. LVOTO was defined as a peak pressure gradient (PG) of LVOT ≥30 mmHg at rest or after provocation on Doppler echocardiography, patients with a LVOT <30 mmHg were referred for physiological exercise to detect the presence of LVOTO in our study (3,14). Baseline data were collected using the hospital information system. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Fuwai Hospital, and all patients provided written informed consent.
Echocardiography
TTE was performed using ultrasonic diagnostic apparatus (EPIQ 7C, Philips Healthcare, Andover, MA, USA) equipped with S5-1 or X5-1 transducers with frequencies ranging from 1 to 5 MHz. Echocardiographic images were analyzed offline using the Qlab 13.0 (Philips Healthcare, Andover, MA, USA). Left ventricular ejection fraction (LVEF), end-diastolic volume (LVEDV), and end-systole volume (LVESV) were measured using biplane Simpson’s method in the apical two-chamber view (A2C) and four-chamber views (A4C). LVEDV and LVESV were indexed according to body surface area (LVEDVi and LVESVi, respectively). The maximal anterobasal IVS thickness was measured in the parasternal long-axis view (PLAX) at end-diastole. The LVOT peak velocity was obtained in the apical three-chamber view (A3C) or five-chamber view (A5C) using continuous-wave Doppler, and the maximal PG was calculated using the Bernoulli equation. Mitral regurgitation (MR) was estimated using color Doppler, and MR was graded based on the native valvular regurgitation guidelines (15): 0, none or trace; 1, mild; 2, moderate; 3, moderate to severe; and 4, severe. SAM was observed in two-dimensional and M-mode echocardiography and was classified as follows: (I) brief anterior motion without mitral-septal contact; (II) brief mitral-septal contact (lasting for <30% of systole); and (III) prolonged mitral-septal contact (lasting for >30% of systole) (16). The lengths of the anterior mitral leaflet (AML) and posterior mitral leaflet (PML) were measured in the A3C at the end of diastole. The mitral valve orifice and ascending aorta (MV-AO) angle was defined as the angle between the plane of the mitral orifice and the ascending aorta, and was measured in the A3C at end-diastole. All the echocardiographic images were acquired and routine measurements were made by two operators with the title of deputy chief physician.
Definition of hypertrophied PM and abnormal MBs
Hypertrophied PM was defined when at least one of the two (anterolateral and posteromedial) PMs had a diameter of >11 mm in the minor axis (8,17). A single muscular band crossing the LV cavity without chordae attached to the MV, but extended from the BS to the PM or apex was defined as abnormal MBs. MBs can be detected in the PLAX, LV short-axis views, A3C, and A4C (6).
Measurement of the basal septal morphology
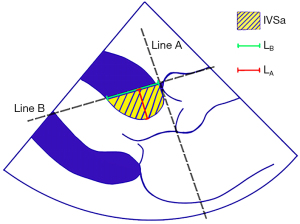
Basal septal morphology was measured offline in the PLAX at the end-systole using the RadiAnt DICOM Viewer (Medixant, Poland) (Figure 2). Measurement of the basal septal morphology was performed by the same operator with five years of professional experience in echocardiography. Line A was obtained by making a straight line from the level of the aortic annulus, and line B passed through the anterior point of the aortic annulus perpendicular to line A. IVSa was defined as the area of basal IVS protruding into the LVOT, which was bounded by lines A and B (if there was a basal MB, then it was included). The IVSa length on line B was defined as LB, and the vertical distance from the deepest point of the IVS that protruded into the LVOT to line B was defined as LA. Standard IVSa (S-IVSa) was defined as IVSa divided by LB.
Statistical analysis
Statistical analyses were performed using SPSS (version 25.0; IBM Inc., Armonk, USA). Categorical variables are presented as n (%). Proportions were compared using the χ2 or Fisher’s exact test. Continuous variables are expressed as mean ± standard deviation when following a normal distribution estimated by the Shapiro-Wilk test, otherwise expressed as median [interquartile range (IQR)]. Continuous variables were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni correction for multiple comparisons or Kruskal-Wallis test. The maximal PG between HOCM and HNOCM patients was compared using a t-test. Echocardiographic parameters associated with LVOTO were analyzed using logistic regression, and the results are described as odds ratios (ORs) with 95% confidence intervals (CIs) and P values. Variables for which the P value was <0.1 in univariable analyses were evaluated in the multivariable model, and backward conditional logistic regression was performed with the models. The correlation between age and MV-AO angle was measured using Pearson’s correlation coefficient. For assessing the reproducibility of the inter-observer and intra-observer measurements, continuous variables were measured by two experienced echocardiographers who were blinded to clinical information. The reproducibility of the inter-observer and intra-observer of IVSa, LA, and LB was assessed using the Bland-Altman method in 20 randomly selected patients. Statistical significance was set at a P value of <0.05.
Results
Patient characteristics
This study recruited 105 HCM patients and 28 healthy controls, and their baseline clinical characteristics are summarized in Table 1. Based on whether the peak LVOT PG was ≥30 mmHg, patients were divided into the obstructive group (HOCM, n=64) and non-obstructive group (HNOCM, n=41). Baseline clinical characteristics were comparable among the three groups (HOCM, HNOCM, and controls) and between the HOCM and HNOCM groups, except for the New York Heart Association (NYHA) class (P<0.001), which was significantly higher in HOCM patients than in HNOCM patients (P<0.001).
Table 1
| Characteristics | HCM (n=105) | Controls (n=28) | P value (entire cohort) | P value (HOCM vs. HNOCM) | |
|---|---|---|---|---|---|
| HOCM (n=64) | HNOCM (n=41) | ||||
| Age (years) | 53.1±11.2 | 48.1±15.4 | 47.6±8.7 | 0.054 | 0.13 |
| Male | 41 [64] | 23 [56] | 14 [50] | 0.41 | 0.42 |
| BSA (m2) | 1.8±0.2 | 1.8±0.2 | 1.7±0.1 | 0.12 | >0.99 |
| History of hypertension | 26 [41] | 20 [49] | – | – | 0.42 |
| Hyperlipidaemia | 28 [44] | 16 [39] | – | – | 0.68 |
| Diabetes mellitus | 8 [13] | 7 [17] | – | – | 0.57 |
| Coronary artery disease | 10 [16] | 11 [27] | – | – | 0.21 |
| Angina | 22 [34] | 16 [39] | – | – | 0.68 |
| Dyspnea | 38 [59] | 27 [66] | – | – | 0.54 |
| Palpitations | 25 [39] | 18 [44] | – | – | 0.91 |
| NYHA class I/II/III/IV, n | 1/21/41/1 | 25/12/4/0† | 28/0/0/0†‡ | <0.001*** | <0.001*** |
| Systolic blood pressure (mmHg) | 127.0±16.6 | 128.2±21.5 | 119.0±9.1 | 0.06 | >0.99 |
| Diastolic blood pressure (mmHg) | 73.4±9.7 | 74.8±11.0 | 78.0±8.2 | 0.12 | >0.99 |
| Family history of HCM | 10 [16] | 9 [22] | – | – | 0.44 |
| History of syncope | 14 [22] | 12 [29] | – | – | 0.48 |
| Beta-blockers | 32 [50] | 21 [51] | – | – | >0.99 |
| Calcium antagonists | 8 [13] | 8 [20] | – | – | 0.40 |
†, P<0.05 vs. HOCM; ‡, P<0.05 vs. HNOCM; ***, P≤0.001. Data are presented as mean ± standard deviation or n [%]. HCM, hypertrophic cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; HNOCM, hypertrophic non-obstructive cardiomyopathy; BSA, body surface area; NYHA, New York Heart Association.
Echocardiographic findings
Echocardiographic results and comparisons between all three groups are shown in Table 2. LVEDVi and LVESVi were smaller in HOCM and HNOCM patients than in controls (P=0.001 and P <0.001, respectively), and LVEF was higher in HOCM patients than in controls (P=0.001). No differences were observed in LVEDVi, LVESVi, LVEF, or in the presence of hypertrophied PMs between HOCM and HNOCM patients (P>0.99, P=0.06, P=0.32, and P=0.12, respectively). The maximal basal septal thickness was higher in HOCM and HNOCM patients than that in controls (P<0.001), and there was no statistical difference between HOCM and HNOCM (P>0.99).
Table 2
| Parameters | HCM (n=105) | Controls (n=28) | P value (entire cohort) | P value (HOCM vs. HNOCM) | |
|---|---|---|---|---|---|
| HOCM (n=64) | HNOCM (n=41) | ||||
| LVEDVi (mL/m2) | 47.5 (38.8–56.0) | 48.6 (41.1–54.1) | 55.9 (50.4–65.0)†‡ | 0.001*** | >0.99 |
| LVESVi (mL/m2) | 11.7 (8.9–14.7) | 13.3 (10.5–16.6) | 20.1 (16.0–22.8)†‡ | <0.001*** | 0.06 |
| LVEF (%) | 69.0 (67.0–73.0) | 68.0 (63.5–71.5) | 64.5 (62.3–68.0)† | 0.001*** | 0.32 |
| Maximal basal septal thickness (mm) | 22.3±3.6 | 22.2±6.1 | 8.7±1.2†‡ | <0.001*** | >0.99 |
| Maximal LVOT PG (mmHg) | 95.1±28.4 | 14.2±6.8† | – | – | <0.001*** |
| Mitral regurgitation grades ≥2 | 54 [84] | 4 [10]† | – | – | <0.001*** |
| SAM grades ≥2 | 47 [73] | 1 [2]† | – | – | <0.001*** |
| Length of AML (mm) | 29.0±2.7 | 25.9±2.5† | 20.3±2.6†‡ | <0.001*** | <0.001*** |
| Length of PML (mm) | 18.7±1.9 | 16.6±1.9† | 12.4±2.0†‡ | <0.001*** | <0.001*** |
| MV-AO angle (°) | 144.6±10.6 | 128.4±7.6† | 126.0±9.7† | <0.001*** | <0.001*** |
| Hypertrophied PMs | 14 [21.9] | 4 [9.8] | – | – | 0.12 |
| Presence of abnormal MBs | 36 [56] | 13 [31]† | – | – | 0.01** |
| IVSa (mm2) | 217.0±68.3 | 171.0±57.3† | 76.8±34.4†‡ | <0.001*** | 0.002** |
| LA (mm) | 10.0±2.1 | 7.7±1.9† | 5.7±1.8†‡ | <0.001*** | <0.001*** |
| LB (mm) | 29.1±5.7 | 29.4±5.4 | 18.7±4.0†‡ | <0.001*** | >0.99 |
| S-IVSa (mm2) | 7.3±1.3 | 5.7±1.2† | 3.9±1.2† | <0.001*** | 0.03* |
Data are presented as mean ± standard deviation, median (interquartile range) or n [%]. †, P<0.05 vs. HOCM; ‡, P<0.05 vs. HNOCM; *, P≤0.05, **, P≤0.01, ***, P≤0.001. HCM, hypertrophic cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; HNOCM, hypertrophic non-obstructive cardiomyopathy; LVEDVi, left ventricular end-diastolic volume indexed to body surface area; LVESVi, left ventricular end-systolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; PG, pressure gradient; SAM, systolic anterior motion; AML, anterior mitral leaflet; PML, posterior mitral leaflet; MV-AO angle, the angle between the mitral valve orifice and ascending aorta; PMs, papillary muscles; MBs, muscle bundles; IVSa, the area of the basal septum protruding into the LVOT; LA, the largest distance of the basal septum protruding into the LVOT; LB, IVSa length in the direction perpendicular to the LA; S-IVSa, IVSa divided by LB.
For dynamic characteristics of LVOT, the maximal LVOT PG in the HOCM group was statistically higher than that in the HNOCM group (P<0.001). MR grade ≥2 (84% vs. 10%, P<0.001) and SAM grade ≥2 (73% vs. 2%, P<0.001) were more common in HOCM patients than in HNOCM patients. Of the 64 HOCM patients, 51 (80%) underwent septal myectomy (SM), and only three patients had a residual gradient >30 mmHg after surgery (64 mmHg with a grade 2 SAM and grade 1 MR; 32 mmHg with a grade 2 SAM and grade 2 MR; and 96 mmHg with a grade 2 SAM and grade 3 MR).
There were statistically significant in the lengths of the AML and PML among HOCM patients, HNOCM patients, and controls (P<0.001 for AML; P<0.001 for PML). AML and PML were significantly longer in HOCM patients than in HNOCM patients (P<0.001). The MV-AO angle was larger in HOCM patients than that in HNOCM patients and controls, and the statistical difference among the three groups was significant (P<0.001). However, no significant difference was observed between the HNOCM and control groups (P=0.92). The MV-AO angle in patients with HCM was positively associated with age (r=0.304, P=0.002). There was no significant correlation between age and MV-AO angle in controls (r=−0.205, P=0.29).
BS morphology
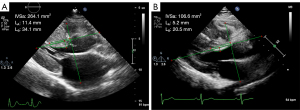
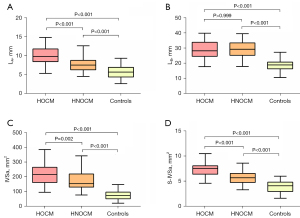
Figure 3 illustrates the measurement of BS morphology in HOCM and HNOCM patients with the same BS thickness. The IVSa, LA and S-IVSa were larger in HOCM and HNOCM patients than in controls (P<0.001 for IVSa; P<0.001, for LA; P<0.001 for S-IVSa), and the differences were significant between the HOCM and HNOCM groups (P=0.002 for IVSa, P<0.001 for LA, and P=0.03 for S-IVSa) (Figure 4). There was a statistically significant in LB among the HOCM, HNOCM, and control groups (P<0.001), but no difference was observed between the HOCM and HNOCM patients (P>0.99).
Echocardiographic parameters associated with LVOTO in patients with HCM
The association between various echocardiographic parameters and LVOTO was assessed using logistic regression analysis (Table 3). Univariable analysis revealed that the AML length, PML length, MV-AO angle, and presence of abnormal MBs, IVSa, LA, and S-IVSa were significantly associated with LVOTO. In the multivariate analysis, a large AML, an increased MV-AO angle, a large IVSa, and a large S-IVSa were associated with LVOTO.
Table 3
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95 % CI) | P value | OR (95 % CI) | P value | ||
| Length of AML | 0.574 (0.452–0.729) | <0.001*** | 0.649 (0.462–0.911) | 0.01** | |
| Length of PML | 0.542 (0.409–0.718) | <0.001*** | |||
| MV-AO angle | 0.843 (0.790–0.898) | <0.001*** | 0.842 (0.768–0.923) | <0.001*** | |
| Presence of abnormal MBs | 2.769 (1.217–6.304) | 0.01** | |||
| Hypertrophied PMs | 2.590 (0.788–8.511) | 0.11 | |||
| IVSa | 0.990 (0.983–0.997) | 0.003** | 1.025 (1.001–1.049) | 0.04* | |
| LA | 0.568 (0.443–0.729) | <0.001*** | |||
| S-IVSa | 0.457 (0.318–0.656) | <0.001 *** | 0.276 (0.101–0.754) | 0.01** | |
*, P≤0.05; **, P≤0.01; ***, P≤0.001. For univariable analysis, results were described as OR with 95% CI and P values. Variables for which the P value <0.1 in univariable analysis were evaluated in the multivariable model. LVOTO, left ventricular outflow tract obstruction; HCM, hypertrophic cardiomyopathy; OR, odds ratio; CI, confidence interval; AML, anterior mitral leaflet; PML, posterior mitral leaflet; MV-AO angle, the angle between the mitral valve orifice and ascending aorta; MBs, muscle bundles; PMs, papillary muscles; IVSa, the area of the basal septum protruding into the LVOT; LA, the largest distance of the basal septum protruding into the LVOT; S-IVSa, IVSa divided by LB (IVSa length in the direction perpendicular to the LA).
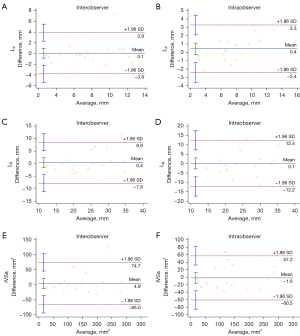
Interobserver and intraobserver reproducibility
The interobserver and intraobserver reproducibility for LA, LB, and IVSa measurements were robust (Figure 5). Bland-Altman analysis showed no significant differences for either intraobserver [mean bias 0.4 mm (95% CI: −0.2 to 1.1) for LA; 0.1 mm (95% CI: −2.8 to 3.1) for LB; and −1.6 mm2 (95% CI: −15.5 to 12.4) for IVSa], or interobserver measurements [mean bias 0.1 mm (95% CI: −0.8 to 1.0) for LA; 0.4 mm (95% CI: −1.6 to 2.93) for LB; and 4.9 mm2 (95% CI: −11.8 to 21.6) for IVSa].
Discussion
Through quantitative assessment, we found that a larger area of IVS protruding into the LVOT was common in HOCM patients, which may influence the morphological characteristics of LVOT by reducing the distance between the IVS and AML, together with the longer AML and increased MV-AO angle, resulting in the facilitation of SAM and LVOTO. In the multivariate analysis, a longer AML, an increased MV-AO angle, a large IVSa, and a large S-IVSa were associated with LVOTO.
LVOTO has been a major characteristic of HCM since it was first pathologically described (1). Although the mechanism leading to this phenomenon has been controversial for many years, the general viewpoint is that the flow-mediated forces on mitral leaflets and/or their supporting apparatus give rise to systolic movement of leaflets towards the IVS, thus inducing LVOTO (18). The two dominant mechanisms responsible for LVOTO are as follows: (I) the hypertrophied septum narrows the LVOT, resulting in abnormal blood flow vectors causing dynamic anterior displacement of the MV leaflets, and (II) anatomical alterations of the MV and apparatus, including elongated leaflets as well as anterior displacement of the PM and MV apparatus, which makes the valve more susceptible to abnormal flow vectors (3). Thus, SAM is generated, leading to LVOTO, high intraventricular PGs, and MR from malcoaptation of the leaflets (3).
BS hypertrophy is a basic feature of HCM and is involved in the occurrence of obstruction in most cases. Nevertheless, researchers have also demonstrated that, in patients with mild BS thickness, LVOTO may be induced by SAM caused by elongated and highly mobile MV leaflets (12). In contrast, in a cohort of unselected patients with isolated basal ventricular septal hypertrophy, LVOTO arose because of the co-existence of MV coaptation and LV hypercontractility, especially the relative position of the MV and LVOT (11). Regardless of the degree of BS thickening, various factors may cause LVOTO. In our cohort of patients with HCM, we focused on a subset of patients with HOCM and HNOCM with a consistent degree of BS thickness. To quantitatively assess the morphology of the BS relative to the LVOT, we propose a novel measurement method for the BS protruding into the LVOT. In our study, we found that the area of IVS protruding into the LVOT (IVSa) and indexed IVSa (S-IVSa) was larger in HCM than in healthy individuals. These indices were larger in patients with HOCM than in those with HNOCM. The LVOT is the region of the left ventricle that lies between the anterior cusp of the MV and the ventricular septum. IVSa and S-IVSa could be regarded as effective hypertrophic areas of IVS because only this part of IVS could have an impact on LVOT morphology and hemodynamics. Thus, we speculate that IVS morphology may be a probable factor in the induction of LVOTO. The morphology of the BS directly affects the morphology of the LVOT. The larger the area of the effective hypertrophied IVS, the more likely it is to alter the blood flow pattern of the LVOT and produce obstruction.
Maron et al. (19) revealed that elongation of the mitral leaflets is a primary phenotypic expression of HCM and a contributor to LVOTO. Numerous studies have reported that mitral leaflets in HOCM patients are longer than those in HNOCM patients (20-22). Our results confirmed that both the AML and PML were longer in HOCM patients than in HNOCM patients, and AML was still found to be associated with LVOTO in the multivariate analysis. The elongated leaflets protruding into the LV cavity are far above the mitral annulus plane (20) and play a significant role in facilitating SAM (23). During late diastole and early systole, blood flow impinges on the posterior surfaces of protruding leaflets at a high angle of attack and pushes them into a position juxtaposed with the IVS (24). After the mitral leaflet contacts the IVS, the PG pushes the obstructed leaflet further towards the IVS (25). Lentz Carvalho et al. (26) conducted a study to explore whether the MV leaflets’ length was associated with the outcomes of SM. They found that the MV leaflets were longer in patients undergoing SM than in patients undergoing coronary artery bypass grafting or aortic valve replacement. Further investigation demonstrated that the postoperative LVOT PG or the relief of the PG was not associated with the length of the AML, which indicated that SM beyond the septal contact point was sufficient in almost all patients without intrinsic MV disease.
Previous studies have demonstrated that “aortoseptal angle” or “left ventricular to aortic root angle” were smaller in HOCM patients than in HNOCM patients, and a steeper left ventricular to aortic root angle is an independent predictor of LVOTO (27,28). These conditions may increase the turbulence of flow from the left ventricle into the aorta and increase intraventricular pressure, thus resulting in LVOT remodeling (28). Unlike previous studies, we chose to measure the angle between the MV annulus and ascending aorta to avoid potential confounding factors that could be generated by varying degrees of septal hypertrophy and inaccurate identification the long axis of the left ventricle. A large MV-AO angle is one of the factors that induce SAM by shifting the mitral leaflets anteriorly (29,30). Consistent with previous findings, our study showed that patients with HOCM had a larger MV-AO angle than those with HNOCM, and this was one of the echocardiographic parameters that was associated with LVOTO. In addition, in patients with HCM, the MV-AO angle appeared to have a positive correlation with age. We speculated that the influence of a larger MV-AO angle on the blood flow pattern was similar to the steeper left ventricular-to-aortic root angle, and LVOT remodeling (represented as a larger MV-AO angle) with age was more obvious in patients with HCM than in healthy individuals.
PM hypertrophy presented in more than half of the patients with HCM. The hypertrophy of BS and PMs may narrow the LVOT by decreasing the distance between the IVS and anterolateral PMs (31). An echocardiography study by Erden et al. (32) found that the cross-sectional area of PMs in patients with HCM was larger than that in healthy patients, and LVOTO occurred with a high frequency in patients with severely hypertrophied PMs. In this study, we observed the incidence of hypertrophied PMs and no difference was found in the presence of hypertrophied PMs between HOCM and HNOCM patients. Irrespective of demographic and clinical characteristics (included septal thickness), the presence of apical MB is associated with HCM expression. The prevalence of apical MBs in patients with mild septal hypertrophy has been demonstrated to be consistent with that in patients with evident septal hypertrophy. Researchers revealed that basal MBs, integrating with the hypertrophied IVS, increased MV-AO angle, and longer AML, may narrow the LVOT and lead to SAM by bringing the IVS/MBs closer to AML (6). In our study, we found that abnormal MBs were more common in patients with HOCM than in those with HNOCM, which is consistent with previous findings. In the univariable logistic regression analysis, the presence of MBs was found to be one of the associated factors for LVOTO, in addition to the length of the AML, PML, MV-AO angle, IVSa, and a large S-IVSa. However, in further multivariable analyses, the presence of MBs was not considered an associated factor for LVOTO. We speculated that the presence of abnormal basal MBs may facilitate LVOTO by increasing IVSa, which was found to be related to LVOTO.
Limitations
This study had several limitations. First, the patients enrolled in our study were from a single center. Second, enrollment of eligible cases was conducted by the same author, resulting in selection bias in our study. Third, the numbers of patients included in this study was is not large enough. Fourth, the definition of LVOTO (PG >30 mmHg) is not a natural categorical status, and a larger sample size to explore the BS morphology and continuous PG of LVOT is still needed. Fifth, there are many recognized risk factors related to LVOTO (such as abnormalities of the mitral apparatus and subvalvular apparatus), but we analyzed only hypertrophy of PMs and did not include other abnormalities such as PM displacement or insertion of PM into the mitral leaflets. Since this was a retrospective study, we could only gather information from the completed examinations. Because of the limited and incomplete data regarding mitral apparatus and subvalvular apparatus, we failed to include more recognized risk factors related to LVOTO. In the future, we would like to conduct a prospective study to collect more detailed information of these risk factors. Finally, morphological changes of the BS in patients with HOCM after SM or alcohol septal ablation have not been estimated; therefore, the role of basal septal morphology in the relief of obstruction is unclear.
Conclusions
Morphological alterations of the BS relative to the LVOT are associated with LVOTO. More attention should be paid to detailed assessing the basal septal morphology and its surrounding structures in addition to basal septal thickness. IVS with a large area protruding into the LVOT was common in patients with HOCM, which may integrate into the elongated AML and large MV-AO angle, resulting in the facilitation of SAM and LVOTO. A longer AML, an increased MV-AO angle, a large IVSa, and a large S-IVSa were associated with LVOTO.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1034/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1034/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Fuwai Hospital, and all patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet 2017;389:1253-67. [Crossref] [PubMed]
- Zhu C, Wang S, Wang S, Meng Y, Yang Q, Nie C, Sun H. Prevalence and characteristics of intramural coronary artery in hypertrophic obstructive cardiomyopathy: a coronary computed tomography and invasive angiography study. Quant Imaging Med Surg 2021;11:162-71. [Crossref] [PubMed]
- Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020;142:e558-631. [PubMed]
- Sherrid MV, Arabadjian M. Echocardiography to individualize treatment for hypertrophic cardiomyopathy. Prog Cardiovasc Dis 2012;54:461-76. [Crossref] [PubMed]
- Parato VM, Antoncecchi V, Sozzi F, Marazia S, Zito A, Maiello M, Palmiero P. Echocardiographic diagnosis of the different phenotypes of hypertrophic cardiomyopathy. Cardiovasc Ultrasound 2016;14:30. [Crossref] [PubMed]
- Xiao M, Nie C, Wang J, Zhu C, Sun X, Zhu Z, Wang H, Wang S. Left ventricular basal muscle bundle in hypertrophic cardiomyopathy: insights into the mechanism of left ventricular outflow tract obstruction. Eur Heart J Cardiovasc Imaging 2022;23:1018-26. [Crossref] [PubMed]
- Silbiger JJ. Abnormalities of the Mitral Apparatus in Hypertrophic Cardiomyopathy: Echocardiographic, Pathophysiologic, and Surgical Insights. J Am Soc Echocardiogr 2016;29:622-39. [Crossref] [PubMed]
- Tao J, Duan F, Long J, Meng Q, Zhang B, Zhu Z, Wang H. The Role of the Submitral Apparatus in Hypertrophic Obstructive Cardiomyopathy. J Am Soc Echocardiogr 2023;36:133-45. [Crossref] [PubMed]
- Chen X, Pan J, Shu J, Zhang X, Ye L, Chen L, Hu Y, Yu R. Prognostic value of regional strain by cardiovascular magnetic resonance feature tracking in hypertrophic cardiomyopathy. Quant Imaging Med Surg 2022;12:627-41. [Crossref] [PubMed]
- Dulguerov F, Marcacci C, Alexandrescu C, Chan KM, Dreyfus GD. Hypertrophic obstructive cardiomyopathy: the mitral valve could be the key. Eur J Cardiothorac Surg 2016;50:61-5. [Crossref] [PubMed]
- Ranasinghe I, Ayoub C, Cheruvu C, Freedman SB, Yiannikas J. Isolated hypertrophy of the basal ventricular septum: characteristics of patients with and without outflow tract obstruction. Int J Cardiol 2014;173:487-93. [Crossref] [PubMed]
- Rowin EJ, Maron BJ, Chokshi A, Kannappan M, Arkun K, Wang W, Rastegar H, Maron MS. Clinical Spectrum and Management Implications of Left Ventricular Outflow Obstruction With Mild Ventricular Septal Thickness in Hypertrophic Cardiomyopathy. Am J Cardiol 2018;122:1409-20. [Crossref] [PubMed]
- Gupta KK, Attri JP, Singh A, Kaur H, Kaur G. Basic concepts for sample size calculation: Critical step for any clinical trials! Saudi J Anaesth 2016;10:328-31. [Crossref] [PubMed]
- Authors/Task Force members. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Raut M, Maheshwari A, Swain B. Awareness of 'Systolic Anterior Motion' in Different Conditions. Clin Med Insights Cardiol 2018;12:1179546817751921. [Crossref] [PubMed]
- Uhm JS, Youn JC, Lee HJ, Park J, Park JK, Shim CY, Hong GR, Joung B, Pak HN, Lee MH. Accessory papillary muscles and papillary muscle hypertrophy are associated with sudden cardiac arrest of unknown cause. Int J Cardiol 2015;197:285-91. [Crossref] [PubMed]
- Morant K, Mikami Y, Nevis I, McCarty D, Stirrat J, Scholl D, Rajchl M, Giannoccaro P, Kolman L, Heydari B, Lydell C, Howarth A, Grant A, White JA. Contribution of mitral valve leaflet length and septal wall thickness to outflow tract obstruction in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 2017;33:1201-11. [Crossref] [PubMed]
- Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40-7. [Crossref] [PubMed]
- Ro R, Halpern D, Sahn DJ, Homel P, Arabadjian M, Lopresto C, Sherrid MV. Vector flow mapping in obstructive hypertrophic cardiomyopathy to assess the relationship of early systolic left ventricular flow and the mitral valve. J Am Coll Cardiol 2014;64:1984-95. [Crossref] [PubMed]
- Halpern DG, Swistel DG, Po JR, Joshi R, Winson G, Arabadjian M, Lopresto C, Kushner J, Kim B, Balaram SK, Sherrid MV. Echocardiography before and after resect-plicate-release surgical myectomy for obstructive hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2015;28:1318-28. [Crossref] [PubMed]
- Venieri E, Aggeli C, Anastasakis A, Sambatakou H, Stefanadis C, Tousoulis D. Mitral valve in hypertrophic cardiomyopathy: a three-dimensional transesophageal study. Hellenic J Cardiol 2021;62:29-34. [Crossref] [PubMed]
- Sherrid MV, Balaram S, Kim B, Axel L, Swistel DG. The Mitral Valve in Obstructive Hypertrophic Cardiomyopathy: A Test in Context. J Am Coll Cardiol 2016;67:1846-58. [Crossref] [PubMed]
- Sherrid MV, Gunsburg DZ, Moldenhauer S, Pearle G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2000;36:1344-54. [Crossref] [PubMed]
- Guigui SA, Torres C, Escolar E, Mihos CG. Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: a narrative review. J Thorac Dis 2022;14:2309-25. [Crossref] [PubMed]
- Lentz Carvalho J, Schaff HV, Nishimura RA, Ommen SR, Geske JB, Lahr BD, Newman DB, Dearani JA. Is anterior mitral valve leaflet length important in outcome of septal myectomy for obstructive hypertrophic cardiomyopathy? J Thorac Cardiovasc Surg 2023;165:79-87.e1. [Crossref] [PubMed]
- Critoph CH, Pantazis A, Tome Esteban MT, Salazar-Mendiguchía J, Pagourelias ED, Moon JC, Elliott PM. The influence of aortoseptal angulation on provocable left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Open Heart 2014;1:e000176. [Crossref] [PubMed]
- Kwon DH, Smedira NG, Popovic ZB, Lytle BW, Setser RM, Thamilarasan M, Schoenhagen P, Flamm SD, Lever HM, Desai MY. Steep left ventricle to aortic root angle and hypertrophic obstructive cardiomyopathy: study of a novel association using three-dimensional multimodality imaging. Heart 2009;95:1784-91. [Crossref] [PubMed]
- Cha JJ, Chung H, Yoon YW, Yoon JH, Kim JY, Min PK, Lee BK, Hong BK, Rim SJ, Kwon HM, Choi EY. Diverse geometric changes related to dynamic left ventricular outflow tract obstruction without overt hypertrophic cardiomyopathy. Cardiovasc Ultrasound 2014;12:23. [Crossref] [PubMed]
- Varghese R, Itagaki S, Anyanwu AC, Trigo P, Fischer G, Adams DH. Predicting systolic anterior motion after mitral valve reconstruction: using intraoperative transoesophageal echocardiography to identify those at greatest risk. Eur J Cardiothorac Surg 2014;45:132-7; discussion 137-8. [Crossref] [PubMed]
- Mutsuga M, Tokuda Y, Fujimoto K, Terazawa S, Ito H, Narita Y, Usui A. Surgery for Anomalous Papillary Muscle Directly Into the Anterior Mitral Leaflet. Ann Thorac Surg 2021;111:1512-8. [Crossref] [PubMed]
- Erden M, van Velzen HG, Menting ME, van den Bosch AE, Ren B, Michels M, Vletter WB, van Domburg RT, Schinkel AFL. Three-dimensional echocardiography for the assessment of left ventricular geometry and papillary muscle morphology in hypertrophic cardiomyopathy. J Ultrasound 2018;21:17-24. [Crossref] [PubMed]


