
Unusual presentation of Rasmussen syndrome affecting both hemispheres at different times
Introduction
Rasmussen syndrome (RS), previously known as Rasmussen encephalitis, is newly defined as etiology-specific epilepsy syndrome by the International League Against Epilepsy Task Force Team (1). It is an uncommon neurological disease characterized by focal seizures with progressive hemiparesis and cognitive impairment (2,3). Most RS cases are reported in childhood, around age six, but adult onset cases have been increasingly reported. Brain biopsies in RS patients have proven multifocal cortical inflammation, neuronal loss, and gliosis in one hemisphere (2). Although the cause of RS is unknown, it is categorized as an etiology-specific epilepsy syndrome because of the pathology obtained from the electroencephalography (EEG) or clinical features (1). Criteria to diagnose RS include the focal seizure or focal motor status epilepticus and brain magnetic resonance imaging (MRI) lesion of hyperintense signal change in the white matter or cortex (2). The clinical disease course is divided into three stages: prodromal, acute, and residual. In the prodromal stage, mild hemiparesis or infrequent seizures might occur, followed by the acute stage with remarkable frequent focal seizures (2). As the disease proceeds chronically, the residual stage with a severe neurological deficit, cognitive decline, motor weakness, and epilepsy can be presented. In this case, we report an unusual presentation of RS involving one hemisphere followed by the other hemisphere.
Case presentation
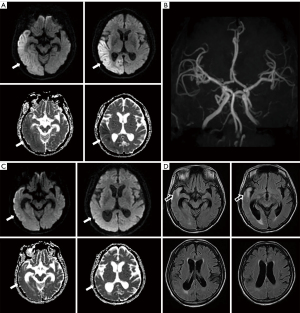
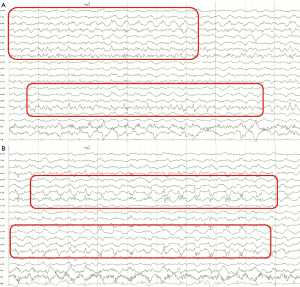
A 58-year-old woman was referred to the emergency room twice in four years because of different neurologic deficits. She reported headache, dysarthria, and drooling in the first attack for three days. A neurological examination showed a mild drowsy mental status with asomatognosia, tactile neglect on the left side, and prosopagnosia. Brain diffusion MRI showed high signal intensities on the right temporal and parietal lobe with mild low signal intensities on the apparent diffusion coefficient (ADC) map (Figure 1A). Brain magnetic resonance angiography (MRA) was normal with intact cerebral arteries (Figure 1B). The lesion in the diffusion and ADC map resolved after one month with a remnant high signal lesion and atrophic change on the right temporal pole and ventricle in the FLAIR magnetic resonance (MR) images (Figure 1C,1D). She required one year of antiseizure medication to become seizure-free. Routine blood tests were within normal limits, except for an increased HbA1C level (9.5%). Central nervous infection and autoimmune encephalitis were excluded based on the cerebrospinal fluid (CSF) profile and autoimmune antibodies. On the 3rd day of hospitalization, the patient had a clonic focal seizure in the left arm and hand accompanied by left facial twitching. EEG on the ictal event was detected by chance that showed fast activities on the right temporo-parietal areas followed by evolution into 1–2 Hz lateralized periodic discharges (Figure 2A,2B). The patient had been administered antiseizure medication to control the focal seizure. She required 45 days of hospitalization and was discharged in an improved condition.
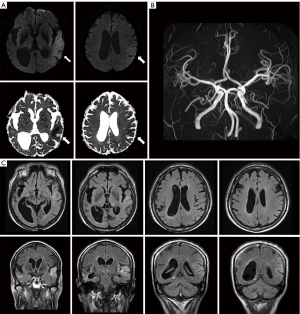
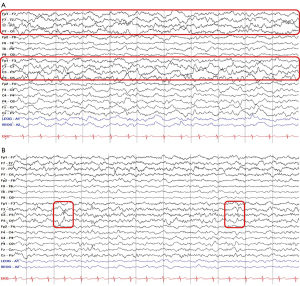
Four years after the first attack, her caregiver reported that the patient had suffered communication difficulties and irritable mood changes for two days. Upon neurological examination, her mentality was drowsy with a reluctant response to verbal commands. She hesitated to speak and showed severe communication problems. Motor power in four extremities was normal, with intact sensory function. Cerebrospinal fluid analysis showed no specific findings, including negative autoimmune encephalitis in NMDA, AMPA, DPPX, LGI1, CASPR2, and GABA-B antibodies. Brain MRI showed diffuse cortical hyperintensity with diffusion restriction in the left temporoparietal lobe (Figure 3A). Bilateral temporal atrophy was observed with asymmetrical enlargement of the right ventricle with normal MRA (Figure 3B,3C). EEG revealed marked asymmetric background rhythm with 7 Hz theta activity in the left and low amplitude 6 Hz theta activity in the right hemisphere (Figure 4A). Frequent episodes of 0.5–1 Hz lateralized rhythmic delta activity and a small amplitude of 1 Hz sharp wave were observed in the left hemisphere (Figure 4A,4B). As the patient had no seizure, she had been administered intravenous methylprednisolone and immunoglobulin for five days without antiseizure medication. Two weeks after admission, she was transferred to another hospital for further evaluation without clear clinical improvement.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Based on the history and clinical characteristics, we confirmed that the patient had two similar neurological deficits in the right and left cortical brain without known etiology in a 4-year interval. The criteria for diagnosing RS can be divided into parts A and B (3). Both cases of this study satisfied part B of the diagnostic criteria for RS. In the first attack, the cortical deficit appeared in the form of asomatognosia, tactile neglect, and prosopagnosia. In the second attack, cortical symptoms appeared as dysphasia involving the dominant hemisphere. This case study differed slightly from the general RS because focal seizures were not observed as initial symptoms in the two acute stages (4-6). The patient experienced the first seizure three days after hospitalization in the first attack. The second attack did not develop a seizure for 9 months, even though lateralized rhythmic delta activity and the sharp wave were observed in the left hemisphere on the EEG.
In this case study, the patient experienced two attacks, and high signal intensity on diffusion weighted image (DWI) and low signal intensity on the ADC map was observed in both episodes. Moreover, DWI/ADC lesions appeared in the right cortex during the first attack and the left cortex during the second attack. These findings suggest cytotoxic edema induced by the limited movement of the water molecules, which means hospitalization events were in the acute phase. There is limited literature about diffusion restriction imaging in the acute stage of RS (7,8). Since diffusion restriction due to cytotoxic edema is a typical finding of ischemic stroke, it is important to differentiate it from RS. It was possible to exclude ischemic stroke for this patient because this patient did not have abnormal intracranial angiography, and the area of high signal intensity on DWI did not match the cerebral artery’s blood supply area. Diagnostic work up of mitochondrial DNA mutation, 14-3-3 brain protein for Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes and Creutzfeldt-Jakob disease were not evaluated due to economic constraints. However, differential diagnosis could be considered as the neurologic symptoms occurred within 2–3 days with rapid disease course followed by residual stage without familial history. At the time of the first attack, the electroclinical seizure was observed at the lesion side during the focal seizure on EEG. At the time of the second attack, background asymmetry was observed on EEG, and nearly continuous lateralized rhythmic delta activity with the sharp wave was found on the left side, which correlated with the location of the brain lesion.
In summary, the patient experienced unilateral hemispheric involvement in the right and left sides over four years. There has been a lack of pathophysiologic mechanisms as to why RS affects only one cerebral hemisphere, and the source of the presumed antigen might estimate it. Possible hypotheses are the involvement of a hemisphere due to a foreign infectious agent or the presence of a gene influencing the generation and formation of one hemisphere, or an autoimmune disease affecting protein (2). Since this patient developed RS at one side each time over 4 years, it was inferred that it occurred in the more vulnerable hemisphere first. The results of this study will serve as a basis for future research to understand the mechanism of RS development. It would be important to consider RS as a differential diagnosis, even if a focal seizure is absent with initial symptoms when there is a clinically clear cortical symptom and there is a lesion on the MRI that is correlated with EEG findings.
Acknowledgments
This case was presented as an abstract in the 41st Annual Autumn Meeting of the Korean Neurological Association in 2022.
Funding: This research was supported by the Basic Science Research Programthrough the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R1I1A3056006).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1031/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riney K, Bogacz A, Somerville E, Hirsch E, Nabbout R, Scheffer IE, Zuberi SM, Alsaadi T, Jain S, French J, Specchio N, Trinka E, Wiebe S, Auvin S, Cabral-Lim L, Naidoo A, Perucca E, Moshé SL, Wirrell EC, Tinuper P. International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022;63:1443-74. [Crossref] [PubMed]
- Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, Vincent A, Mathern GW, Cross JH. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol 2014;13:195-205. [Crossref] [PubMed]
- Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, Kurthen M, Lassmann H, Mantegazza R, Villemure JG, Spreafico R, Elger CE. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 2005;128:454-71. [Crossref] [PubMed]
- Kupila L, Jutila L, Immonen A, Vanninen R, Mervaala E, Pateau A, Luostarinen L, Kälviäinen R. Late-onset Rasmussen’s encephalitis and long-term remission. Epileptic Disord 2011;13:88-91. [Crossref] [PubMed]
- Dupont S, Gales A, Sammey S, Vidailhet M, Lambrecq V. Late-onset Rasmussen Encephalitis: A literature appraisal. Autoimmun Rev 2017;16:803-10. [Crossref] [PubMed]
- Bien CG, Widman G, Urbach H, Sassen R, Kuczaty S, Wiestler OD, Schramm J, Elger CE. The natural history of Rasmussen’s encephalitis. Brain 2002;125:1751-9. [Crossref] [PubMed]
- Furruqh F, Thirunavukarasu S, Biswas A, Vivekandan R. Complete right cerebral hemispheric diffusion restriction and its follow-up in a case of Rasmussen’s encephalitis. BMJ Case Rep 2015;2015:bcr2015212256. [Crossref] [PubMed]
- Pradeep K, Sinha S, Saini J, Mahadevan A, Arivazhagan A, Bharath RD, Bindu PS, Jamuna R, Rao MB, Chandramouli BA, Shankar SK, Satishchandra P. Evolution of MRI changes in Rasmussen’s encephalitis. Acta Neurol Scand 2014;130:253-9. [Crossref] [PubMed]


