
Imaging biomarkers on Angio-CT for predicting the efficacy of transarterial chemoembolization in hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cancer, with a cancer-related mortality rate of 8.2% worldwide (1). Resection, liver transplantation, and local ablation are the main treatment for early stage HCC, but most of the liver cancer is found in the intermediate and advanced stages, and the above treatment methods are not suitable (2,3). Trans-arterial therapies, including conventional TACE (c-TACE), drug-eluting beads TACE (D-TACE), and transarterial radioembolization (TARE), is the standard treatment for intermediated and advanced HCCs, that can significantly prolong the survival time (4). The c-TACE, referred to as the most widely used embolization method (5), can block the tumor blood supply by using chemotherapeutic agents mixed with the lipiodol and injecting into the tumor feeding artery of the hepatic artery (6). The efficacy of TACE has been evaluated according to the modified Response Evaluation Criteria In Solid Tumors (mRECIST) standards that complete response (CR) in therapeutic efficacy has been assessed according to the tumor enhancement status in computed tomography (CT) and/or magnetic resonance (MR) (7). However, the therapeutic response to TACE varies among individuals with a single HCC nodule (8). Therefore, it is warranted to identify appropriate individuals for TACE by finding indicators to predict the efficacy of TACE.
Early assessment of the TACE efficacy has been necessary for successful treatment by determining predictors for response to TACE in HCC nodules. Many readily available imaging resources are potentially informative and several studies have confirmed correlation between imaging biomarkers and TACE efficacy (9-17). Delayed percentage attenuation ratio (DPAR), defined as the relationship between the attenuation of the adjacent liver parenchyma and the attenuation of the tumor area, is strongly associated with CR of a single HCC nodule after TACE that DPAR ≥120 could perfectly predict tumor necrosis (9). The lipiodol coverage rate and tumor volume were associated with the efficacy of TACE based on multi-detector CT (MDCT) after TACE (10,11). Tumor margins, size, and location have also been considered good predictors for TACE efficacy. The presence of smoother margins, smaller size, and better location of HCC nodule resulted in better efficacy (12-14). Lipiodol retention pattern, detected based on cone beam CT (CBCT), has been found significant value in predicting response to TACE that tumors with good lipiodol deposition were associated with short-term response (15-17).
Angio-CT during hepatic arteriography has been used to find HCC nodules when performing TACE, which has been proven to be more sensitive than CBCT in detecting tumors (18). However, few studies have used Angio-CT to predict the efficacy of TACE, which only focused on tumor margins and lipiodol retention patterns (19,20). The efficacy of Angio-CT demands full exploration by investigating the potential of other imaging biomarkers.
Our study aimed to identify new imaging biomarkers on Angio-CT, including new lipiodol retention patterns, the imaging biomarkers on Angio-CT during hepatic arteriography, and lipiodol deposition density to predict the efficacy of TACE using software-based segmentation in patients with HCC receiving c-TACE.
Methods
Patients
This retrospective study enrolled patients with advanced HCC nodules treated with c-TACE from June 2021 to June 2022. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bioethics Committee of Beijing Friendship Hospital (No. 2022-P2-282-01). During the admission period of TACE, informed consent was registered from each patient. Informed consent for this study was also obtained from patients. All patients’ baseline and imaging information was stored in a database used for the study. The HCC was diagnosed according to dynamic CT or MR imaging and/or pathological specimens by biopsy. The inclusion criteria were as follows: (I) age ≥18 years; (II) Eastern Cooperative Oncology Group (ECOG) score of 0–2; (III) Child-Pugh class of A or B. Patients were excluded if they had received c-TACE treatment previously.
TACE procedure
The c-TACEs were performed using an angiograph system (Artis ZEE Ⅲ Ceiling, SIEMENS Healthineers) by interventional radiologists with at least ten years of experience. A 5-French angiography catheter (RH type, Cook) was inserted through the femoral artery to the celiac trunk. Digital subtraction angiography was performed in the celiac trunk to show the whole liver blood flow and the distribution of intrahepatic nodules. Arteriography in the superior mesenteric artery was used to show the presence of an accessory hepatic artery. The superselective choice was performed carefully if extrahepatic arteries were involved in the tumor blood supply. A 2.6 Fr microcatheter (Stride, ASAKI) was used to superselectively choose the tumor artery. Each artery was carefully embolized for cases with multiple feeding arteries. Chemoembolization was performed using 5–15 mL lipiodol (Guerbet, Aulnay-sous-Bois) mixed with 40–60 mL epirubicin (Hisun Pharmaceutical), which was slowly injected into the tumor under fluoroscopy. The endpoint of embolization was defined as lipiodol retention in the tumor feeding artery and/or peripheral portal vein development. Gelfoam (Alicon Pham SCI &TEC) was finally injected into the tumor. We usually choose gel foam with a particle size of 150–350 µm. A repeated celiac trunk angiography was usually performed to determine the efficacy of c-TACE.
Angio-CT examinations
Angio-CT examinations were performed with a CT system (Syngo CT VA62A, SIEMENS Healthineers). This examination was usually performed after superselective choice to the tumor feeding artery and the end of TACE. Angio-CT after superselective choice during hepatic arteriography was used to determine tumor location and analyze tumor enhancement. A contrast medium was injected through a microcatheter. The scanning was performed according to the previous protocol (18); the injection rate was 1 mL/s with a 4-second delay to obtain arterial phase images. The scanning protocol was: a 5-mm slice thickness; 40 rows detector; 1-mm reconstruction intervals. The plain CT at the end of c-TACE was conducted to determine the lipiodol deposition.
Outcome evaluation and image analysis
All patients underwent enhanced CT before c-TACE and plain CT one month after the TACE to determine the deposition of iodized oil. At the same time, enhanced MR and/or CT were performed to analyze the efficacy of TACE by two experienced radiologists according to the mRECIST standard. The CR was identified as CR, and the non-CR was defined as partial response (PR), stable disease (SD), and progressive disease (PD). We segmented tumors and calculated the mean CT value by ITK-SNAP software (Version3.6.0, Copyright © 2007 Free Software Foundation, Inc.), which provided a graphical user interface for manual and user-guided semi-automatic segmentation of 3D medical imaging datasets (20). The regions of interest (ROI) were drawn in axial, coronal, and sagittal positions to segment the tumor.
Statistical analysis
The baseline characteristics of the study population were summarized. For continuous variables, mean value and range were evaluated, and for categorical variables, count and proportion were assessed. A Chi-squared test was used to compare the difference in composition ratio and odds ratio between the two groups. Bonferroni correction was also performed. The t-test was used to analyze the mean value of the two groups. Statistical analyses were performed using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA). The confidence interval (CI) was evaluated within the 95% range. P<0.05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics. A total of 45 patients with 72 HCC nodules were enrolled in our retrospective study. All patients underwent Angio-CT examinations during c-TACE. There were 35 men and 10 women with a mean age of 65.21±9.975 years old. The counts of HCC nodules ≤3 and >3 cm were 45 and 27, respectively.
Table 1
| Characteristic | Data (N=45) |
|---|---|
| Age (years) | 65.21±9.975 |
| Sex (M/F) | 35/10 |
| ECOG score | |
| 0 | 37 |
| 1 | 8 |
| Liver function | |
| Child-Pugh A | 33 |
| Child-Pugh B | 12 |
| Etiology | |
| Hepatitis B/C | 28 |
| Other | 17 |
| AFP level (ng/mL) | |
| <400 | 12 |
| ≥400 | 33 |
| PIVIKA-Ⅱ level (mAu/mL) | |
| ≤40 | 10 |
| >40 | 35 |
| BCLC stage | |
| B | 28 |
| C | 17 |
| Number of tumors | 72 |
| Tumor size (cm) | |
| ≤3 | 45 |
| >3 | 27 |
| Combination therapy | |
| Only TACE | 23 |
| TACE + TKI | 17 |
| TACE + TKI + PD-1 | 5 |
M, male; F, female; ECOG, Eastern Cooperative Oncology Group; AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer staging system; TKI, tyrosine kinase inhibitor; PD-1, programmed death 1.
Lipiodol deposition pattern in the prediction of the c-TACE efficacy
We first used the lipiodol deposition as an imaging biomarker to predict the treatment efficacy and applied Angio-CT to evaluate intra-TACE iodized oil deposition and plain CT to determine the lipiodol deposition at the end of TACE. Three lipiodol deposition models were set in our study based on the imaging manifestations of lipiodol deposition in treatment: (I) the first type was complete in that HCC nodules were fulfilled with dense and uniform lipiodol; (II) the second type was partial-complete with dense and ground glass-like places; (III) the third type was incomplete that the whole tumor was like ground glass (Figure 1). The formation of ground glass was attributed to little lipiodol injected into the tumor and the low lipiodol deposition. The three lipiodol deposition models on Angio-CT are shown in Figure 1.
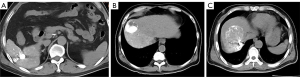
There were 28 nodules enrolled in the complete group, 31 nodules enrolled in the partial-complete group, and 13 nodules enrolled in the incomplete group. In the complete deposition pattern, 18 tumors (64.3%) showed CR, and ten tumors (36.7%) showed non-CR. Two tumors (6.5%) were identified with CR, and 29 (93.5%) were identified with non-CR in the partial-complete deposition pattern. Among the incomplete deposition group, there was one tumor (8.3%) with CR and 11 (91.7%) with non-CR. The CR proportion was significantly higher in patients with good lipiodol deposition (complete) compared with that in patients with poor lipiodol deposition (partial-complete + incomplete) (P<0.0001, OR =24.6, 95% CI: 6.041–100.176) (Table 2).
Table 2
| Characteristic | CR | Non-CR | OR | P value | 95% CI |
|---|---|---|---|---|---|
| Complete deposition | 18 | 10 | 24.6 | <0.0001 | 6.041–100.176 |
| Partial-complete deposition | 2 | 29 | – | – | – |
| Incomplete deposition | 1 | 12 | – | – | – |
CR, complete response; OR, odds ratio; CI, confidence interval.
Lipiodol deposition density in the prediction of the efficacy of c-TACE under a complete deposition pattern
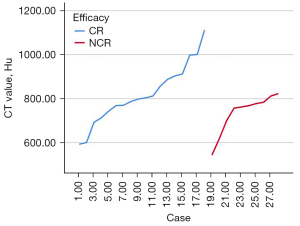
We used ITK-SNAP to segment lipiodol deposition and calculate the mean CT value in the complete deposition group. Semi-automatic and enlarged segmentations can also be used for cases with irregular shapes (Figure 2). Three-dimensional lipiodol deposition was obtained with a mean CT value of 789.13±123.87 HU. Based on the mean CT value, patients were divided into two groups, CT value ≥800 HU and CT value <800 HU. There was no significantly different proportion of CR between CT value ≥800 HU and CT value <800 HU (P=0.119, OR =4.000, 95% CI: 0.659–24.297) (Table 3). The trend showed that the therapeutic efficacy of c-TACE was improved with the mean CT values (Figure 3).
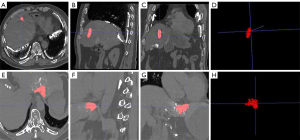
Table 3
| Characteristic | CR | Non-CR | OR | P value | 95% CI |
|---|---|---|---|---|---|
| CT value ≥800 HU | 9 | 12 | 4.000 | 0.119 | 0.659–24.297 |
| CT value <800 HU | 9 | 8 | – | – | – |
CT, computed tomography; CR, complete response; Non-CR, not complete response; OR, odds ratio; CI, confidence interval.
Hepatic vein development in arterial phase on Angio-CT in the prediction of the efficacy of c-TACE under complete deposition pattern
In the complete lipiodol deposition group, Angio-CT results indicated some HCC nodules in hepatic vein development, which were not shown in the DSA image during hepatic arteriography (Figure 4). There were seven cases with hepatic vein development in the arterial phase presence of non-CR. The non-CR rate of these seven cases was significantly different from that of the other 21 cases with no hepatic vein development in the arterial phase) (P<0.0001, OR =0.143, 95% CI: 0.143–0.407) (Table 4). Then, we compared the mean CT values of lipiodol deposition on Angio-CT during c-TACE and plain CT one month after operation between the hepatic vein development and no hepatic vein development groups. In the hepatic vein development, the mean CT value on Angio-CT was 743.34±83.32 HU, and the mean CT value on plain CT one month after the operation was 202.82±59.36 HU. In the no hepatic vein development group, the mean CT value on Angio-CT was 799.63.34±131.02 HU, and the mean CT value on plain CT one month after the operation was 761.20±185.42 HU. There was a significant statistical difference in the change of mean CT values between hepatic vein development and no hepatic vein development groups (Figure 5).
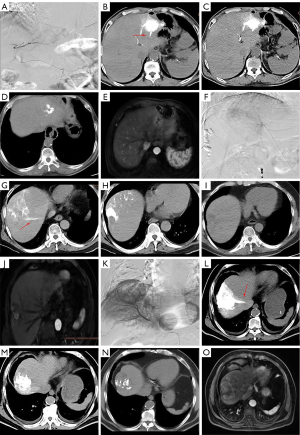
Table 4
| Characteristic | CR | Non-CR | OR | P value | 95% CI |
|---|---|---|---|---|---|
| Non-hepatic vein in artery phase | 18 | 3 | 0.143 | <0.0001 | 0.143–0.407 |
| Hepatic vein in artery phase | 0 | 7 | – | – | – |
CR, complete response; Non-CR, not complete response; OR, odds ratio; CI, confidence interval.

Comparison of multiple imaging biomarkers to predict the efficacy
Various known imaging biomarkers were used to predict therapeutic efficiency in the 28 cases under complete deposition patterns. We only found hepatic vein development on Angio-CT in the artery phase as a significant imaging biomarker that cases with hepatic vein development tended to perform poor c-TACE efficacies (Table 5).
Table 5
| Characteristic | CR | Non-CR | OR | P | 95% CI |
|---|---|---|---|---|---|
| Obvious contrast enhancement | 12 | 4 | 1.333 | 0.724 | 0.269–6.606 |
| Non obvious contrast enhancement | 6 | 6 | – | – | – |
| Clear boundary | 8 | 5 | 0.8 | 0.778 | 0.170–3.767 |
| Unclear boundary | 10 | 5 | – | – | – |
| Tumor size ≥3 cm | 7 | 3 | 1.485 | 0.638 | 0.285–7.743 |
| Tumor size <3 cm | 11 | 7 | – | – | – |
| Superselective choice | 14 | 6 | 2.333 | 0.318 | 0.433–12.568 |
| Non superselective choice | 4 | 4 | – | – | – |
| Good tumor location | 12 | 5 | 2 | 0.387 | 0.412–9.712 |
| Poor tumor location | 6 | 5 | – | – | – |
| DPAR ≥120 | 11 | 3 | 3.667 | 0.115 | 0.703–19.120 |
| DPAR <120 | 7 | 7 | – | – | – |
| lipiodol deposition in portal vein | 12 | 3 | 4.667 | 0.062 | 0.878–24.796 |
| Non lipiodol deposition in portal vein | 6 | 7 | – | – | – |
| CT value of lipiodol ≥800 HU | 9 | 2 | 4 | 0.119 | 0.659–24.297 |
| CT value of lipiodol <800 HU | 9 | 8 | – | – | – |
| Hepatic vein in artery phase | 18 | 3 | 0.143 | <0.0001* | 0.143–0.407 |
| No Hepatic vein in artery phase | 0 | 7 | – | – | – |
| Multiple blood supply of tumor | 7 | 3 | 1.485 | 0.638 | 0.285–7.743 |
| Single blood supply of tumor | 11 | 7 | – | – | – |
*, there were significant differences in two groups. P=0.000042<0.0025 (Bonferroni correction). Noticeable contrast enhancement: the tumors in the arterial phase were significantly enhanced; Clear boundary: the tumor has a complete capsule, no burr, and no surrounding invasion; Good tumor location: tumors in hepatic segments 1, or if the tumor margin was within 1 cm of the liver dome; DPAR defined as the relationship between the attenuation of the adjacent liver parenchyma and the attenuation of the tumor area. c-TACE, conventional transarterial chemoembolization; DPAR, delayed percentage attenuation ratio; CT, computed tomography; CR, complete response; Non-CR, not complete response; OR, odds ratio; CI, confidence interval.
Discussion
In the present study, complete lipiodol deposition on Angio-CT during c-TACE was a predictive factor for therapeutic response to TACE. For HCC nodules with well-deposited lipiodol, hepatic vein development on Angio-CT in the arterial phase was strongly associated with poor efficacy of TACE. There was no statistically difference between the CT value and the therapeutic efficacy of nodules, but the therapeutic efficacy tended to be better when the mean CT values were higher.
As an essential treatment for advanced HCC (4), it is vital to predicting the efficacy of TACE as early as possible. It is a key trigger in predicting therapeutic efficacy to consider whether the whole tumor is included in the TACE procedure or not (21). For HCC nodules without being wholly included in the TACE target, the efficacy of TACE will be incomplete, leading to early local recurrence (22). Therefore, previous articles have explored the lipiodol deposition situation and its associations with the efficacy of TACE (15,16). Hu et al. divided the deposition into three categories depending on the lipiodol covering pattern in tumor regions, including complete (more than 90 %, no peripheral defects), moderate (50-90 %, some with or without peripheral defects), and poor (less than 50 %). The complete group indicated a higher CR rate (15). Tsai et al. evaluated the parenchyma-to-lipiodol ratio (PLR) and lesion-to-lipiodol ratio (LLR) on CBCT to predict 1-year tumor response in patients with HCC treated with c-TACE. The PLR with the optimal cut-off value was found as an objective, effective, and available predictor for 1-year HCC progression after c-TACE (16). The classification of lipiodol deposition in our study was different from the standards in the past. We thought there might be fewer possibilities in the absence of lipiodol deposition especially considering the requirements of precise TACE and the application of Angio-CT. Some HCC nodules might represent poor lipiodol deposition due to other factors (23), including vasospasm, small feeding supply artery, reanalyzed feeding artery, etc. The ground glass deposition pattern (incomplete) would be formed when a small amount of lipiodol was injected into the tumor with poor therapeutic effects compared with the tumor with complete lipiodol deposition, just like the previous study. Previous studies have tried to increase the density of lipiodol through Balloon-occluded TACE (B-TACE) (24-26). B-TACE has been developed in Japan reported by Irie et al. (24). When B-TACE occluded the blood supply artery through the balloon catheter, the lipiodol—chemotherapy drug emulsion was injected. The occlusion of the blood supply artery leads to the change of the internal pressure between the tumor and the liver parenchyma, which makes the lipiodol emulsion accumulate in the target node. According to previous reports, the therapeutic effect of B-TACE is better than that of C-TACE (25,26).
Our study found that among the 28 HCC nodules with complete lipiodol deposition, there were still 10 cases with the therapeutic efficacy of non-CR. We thought it might be related to the lipiodol deposition density at first. A previous study used CT images after several weeks to analyze lipiodol deposition by delineating the ROI to calculate CT values on the two-dimensional level (27). The results from the previous study might be inaccurate due to the delayed CT examination lack of prediction significance and the two-dimensional ROIs hard to reflect the actual lipiodol deposition density of the whole tumor. Previous studies had conducted three-dimensional segmenting of lipiodol deposition by comparing the volume of lipiodol deposition during the TACE and calculating the tumor volume in the follow-up images after the TACE (10,11,17). However, there might also be a lack of representativeness in deposition distribution considering the uneven intra-tumoral deposition density of lipiodol. In our study, only 28 lesions with complete lipiodol deposition were investigated with the consistent distribution of lipiodol. The three-dimensional ROIs of lipiodol deposition were segmented by calculating the mean CT values using ITK-SNAP. Otherwise, the present images were obtained from Angio-CT during TACE, ensuring the prediction indicators’ timeliness. Although our result did not find significantly different proportions of CR between CT values ≥800 HU group and CT values <800 group, the trend was identified that the higher the CT value was, the better the efficacy achieved, which might attribute to the reflection of embolization degree in lipiodol density for the specific distribution range of lipiodol. When the CT value exceeded 800 HU, the efficacy of TACE reached CR. To our knowledge, it was the first time to segment three-dimensional images under Angio-CT by analyzing lipiodol density to predict the efficacy of TACE.
Among these 28 cases with complete lipiodol deposition, we found that the tumor with hepatic vein development in Angio-CT arterial phase reached non-CR regardless of lipiodol deposition. There were a total of 7 tumors with hepatic vein development in the arterial phase evaluated with the efficacy of non-CR. Among various imaging-associated biomarkers, only hepatic vein development was strongly associated with the efficacy of TACE, which might be caused by the presence of a small hepatic artery and hepatic venous fistulas inner tumor. These hepatic arteriovenous fistulas are usually small in diameter or do not open naturally. However, it will open when injecting the high-pressure syringe into the contrast medium (28). In the present study, no hepatic vein development could be seen in the DSA images because of the different image resolutions (Figure 4A,4F,4K). However, Angio-CT could show the hepatic vein development caused by these hepatic arteriovenous fistulas (Figure 4B,4G,4L). Angio-CT after TACE showed no lipiodol deposition in the hepatic vein (Figure 4C,4H,4M) because these arteriovenous fistulas close with a small diameter. With time, the fragmented lipiodol would flow through the hepatic arteriovenous fistula, and the intra-tumoral lipiodol deposition would be significantly reduced (Figure 4D,4I,4N). Thus, the therapeutic effect of TACE was below the mark because there was only a limited amount of lipiodol hard to cause ectopic embolization reaction.
To our knowledge, this was the first time Angio-CT was applied to evaluate the imaging marker associated with potential hepatic arteriovenous fistula to predict TACE efficacy. This study was also the first time to segment lipiodol deposition under Angio-CT and analyze lipiodol density to predict the efficacy of TACE. The main limitation was the small sample size in this study, which needs further validation by large-sample research.
Conclusions
Complete lipiodol deposition on Angio-CT during c-TACE was a predictive factor for therapeutic response to TACE. For HCC nodules with well-deposited lipiodol, hepatic vein development on Angio-CT in the arterial phase was strongly associated with poor efficacy of TACE. Finally, the therapeutic efficacy improved as the mean CT values were raised. The findings in this study need further verification by large-sample research.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1355/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bioethics Committee of Beijing Friendship Hospital (No. 2022-P2-282-01). During the admission period of TACE, informed consent was registered from each patient. Informed consent for this study was also obtained from patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" J Hepatol 69 (2018) 182-236. J Hepatol 2019;70:817. [Crossref] [PubMed]
- Gaba RC, Lewandowski RJ, Hickey R, Baerlocher MO, Cohen EI, Dariushnia SR, Janne d'Othée B, Padia SA, Salem R, Wang DS, Nikolic B, Brown DBSociety of Interventional Radiology Technology Assessment Committee. Transcatheter Therapy for Hepatic Malignancy: Standardization of Terminology and Reporting Criteria. J Vasc Interv Radiol 2016;27:457-73. [Crossref] [PubMed]
- Takayasu K. Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: recent progression and perspective. Oncology 2013;84:28-33. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D'Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011;53:1580-9. [Crossref] [PubMed]
- Fronda M, Doriguzzi Breatta A, Gatti M, Calandri M, Maglia C, Bergamasco L, Righi D, Faletti R, Fonio P. Quantitative assessment of HCC wash-out on CT is a predictor of early complete response to TACE. Eur Radiol 2021;31:6578-88. [Crossref] [PubMed]
- Letzen BS, Malpani R, Miszczuk M, de Ruiter QMB, Petty CW, Rexha I, Nezami N, Laage-Gaupp F, Lin M, Schlachter TR, Chapiro J. Lipiodol as an intra-procedural imaging biomarker for liver tumor response to transarterial chemoembolization: Post-hoc analysis of a prospective clinical trial. Clin Imaging 2021;78:194-200. [Crossref] [PubMed]
- Wang Z, Lin M, Lesage D, Chen R, Chapiro J, Gu T, Tacher V, Duran R, Geschwind JF. Three-dimensional evaluation of lipiodol retention in HCC after chemoembolization: a quantitative comparison between CBCT and MDCT. Acad Radiol 2014;21:393-9. [Crossref] [PubMed]
- Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, Ingrand P, Tasu JP. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol 2016;26:1640-8. [Crossref] [PubMed]
- Zhang W, Xu AH, Wang W, Wu YH, Sun QL, Shu C. Radiological appearance of hepatocellular carcinoma predicts the response to trans-arterial chemoembolization in patients undergoing liver transplantation. BMC Cancer 2019;19:1041. [Crossref] [PubMed]
- Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 2014;203:W253-9. [Crossref] [PubMed]
- Hu J, Maybody M, Cao G, Wang X, Chen H, Zhu X, Yang R, Wang X. Lipiodol retention pattern assessed by cone beam computed tomography during conventional transarterial chemoembolization of hepatocellular carcinoma: accuracy and correlation with response. Cancer Imaging 2016;16:32. [Crossref] [PubMed]
- Tsai YC, Shih JH, Hwang HE, Chiu NC, Lee RC, Tseng HS, Liu CA. Early prediction of 1-year tumor response of hepatocellular carcinoma with lipiodol deposition pattern through post-embolization cone-beam computed tomography during conventional transarterial chemoembolization. Eur Radiol 2021;31:7464-75. [Crossref] [PubMed]
- Wang Z, Chen R, Duran R, Zhao Y, Yenokyan G, Chapiro J, Schernthaner R, Radaelli A, Lin M, Geschwind JF. Intraprocedural 3D Quantification of Lipiodol Deposition on Cone-Beam CT Predicts Tumor Response After Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2015;38:1548-56. [Crossref] [PubMed]
- Taiji R, Lin EY, Lin YM, Yevich S, Avritscher R, Sheth RA, Ruiz JR, Jones AK, Chintalapani G, Nishiofuku H, Tanaka T, Kichikawa K, Gupta S, Odisio BC. Combined Angio-CT Systems: A Roadmap Tool for Precision Therapy in Interventional Oncology. Radiol Imaging Cancer 2021;3:e210039. [Crossref] [PubMed]
- Tajima T, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Kakihara D, Honda H. Safety margins of hepatocellular carcinoma demonstrated by 3-dimensional fused images of computed tomographic hepatic arteriography/unenhanced computed tomography: prognostic significance in patients who underwent transcatheter arterial chemoembolization. J Comput Assist Tomogr 2010;34:712-9. [Crossref] [PubMed]
- Fujita T, Ito K, Tanabe M, Yamatogi S, Sasai H, Matsunaga N. Iodized oil accumulation in hypervascular hepatocellular carcinoma after transcatheter arterial chemoembolization: comparison of imaging findings with CT during hepatic arteriography. J Vasc Interv Radiol 2008;19:333-41. [Crossref] [PubMed]
- Li M, Jiang M, Zhang G, Liu Y, Zhou X. Prediction of fluid intelligence from T1-w MRI images: A precise two-step deep learning framework. PLoS One 2022;17:e0268707. [Crossref] [PubMed]
- Ishimaru H, Nakashima K, Sakugawa T, Sakamoto A, Matsuoka Y, Ashizawa K, Uetani M. Local recurrence after chemoembolization of hepatocellular carcinoma: uptake of gadoxetic acid as a new prognostic factor. AJR Am J Roentgenol 2014;202:744-51. [Crossref] [PubMed]
- Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014;87:22-31. [Crossref] [PubMed]
- Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol 2013;36:706-13. [Crossref] [PubMed]
- Arai H, Abe T, Takayama H, Toyoda M, Ueno T, Kakizaki S, Sato K. Safety and efficacy of balloon-occluded transcatheter arterial chemoembolization using miriplatin for hepatocellular carcinoma. Hepatol Res 2015;45:663-6. [Crossref] [PubMed]
- Irie T, Kuramochi M, Kamoshida T, Takahashi N. Selective balloon-occluded transarterial chemoembolization for patients with one or two hepatocellular carcinoma nodules: Retrospective comparison with conventional super-selective TACE. Hepatol Res 2016;46:209-14. [Crossref] [PubMed]
- Haubold J, Ludwig JM, Li Y, Buechter M, Wetter A, Umutlu L, Theysohn JM. Measuring the density of iodine depositions: Detecting an invisible residual tumor after conventional transarterial chemoembolization. PLoS One 2020;15:e0227972. [Crossref] [PubMed]
- Cai Z, Ran M, Song J, Zhen W, Li M. Imaging Diagnosis and Interventional Treatment for Hepatocellular Carcinoma Combined with Arteriovenous Fistula. J Healthc Eng 2021;2021:6651236. [Crossref] [PubMed]


