Hirayama’s disease: an Italian single center experience and review of the literature
Introduction
Hirayama’s disease (HD), also known as “juvenile muscular atrophy of distal upper extremity” (JMADUE), was first described by Hirayama et al. in 1959 (1). Since then, the greatest number of cases have been reported from Japan and other Asian countries (1-6); in contrast, it is less common among the population of the North America and Europe, however lately some case series were published (7-10). Some case reports were reported in Italy (11-13) but to our knowledge this is the first reported Italian group of patients with HD.
The disease affects young people, predominantly men, in the second to third decades of life and it is characterized by an insidious onset, unilateral or bilateral asymmetric (rarely bilateral symmetric) weakness and atrophy of the forearm and hand with sparing of the brachioradialis muscle giving the characteristic appearance of oblique amyotrophy (14).
HD is a benign, self-limiting pathology; after a progressive phase of the neurological deficits affecting the C7, C8 and T1 myotomes for about 1–5 years, it has a spontaneous arrest (15). The first autopsy obtained in 1982 from a patient died of lung carcinoma, revealed antero-posterior flattening and ischemic changes in the anterior horn cells of the lower cervical cord segment (16).
HD is thought to be a flexion myelopathy related to forward displacement of the posterior wall of the lower cervical dural canal with neck flexion, but this theory is strongly debated (12,17). HD is predominantly sporadic and familial occurrence is very rare, but documented (1,17). Magnetic resonance imaging (MRI) features have been described in literature to aid the diagnosis of HD. In the present study we report our experience of HD and review the clinical, electrophysiological and MRI features, in both neutral and neck flexed position, of a group of Italian patients. Finally we propose an optimized MRI protocol for patients with suspected or diagnosed HD in order to make an early diagnosis and a standardized follow up.
Methods
Eight patients (6 males, 2 females; age range 15–23 years) with clinical suspicion of Hirayama disease underwent clinical, electrophysiological and MRI diagnostic evaluation between January 2007 and November 2013 at University Hospital Federico II, Naples, Italy. We obtained written informed consent from all of our patients.
Diagnostic inclusion criteria for HD were (18,19): (I) weakness and atrophy of the upper limbs interesting predominantly forearms and hands; (II) unilateral or bilateral asymmetric signs and symptoms; (III) insidious onset in the teens or early 20’s; (IV) absence of substantial sensory and reflexes abnormalities of pyramidal tracts, lower limbs, cranial nerves, sphinterial or cerebellar deficits; (V) progression for few years followed by arrest of the disease; (VI) electromyography (EMG) evidence of the chronic denervation at C7-T1 myotomes; (VII) exclusion of other diseases.
MRI studies of cervical spine (C-spine) were conducted with a 1.5 Tesla MRI scanner (Intera, Philips, Best, The Netherlands) in the neutral position as follows: sagittal T1-weighted sequences [turbo spin echo, repetition time (TR) ms/echo time (TE) ms of 400/10], sagittal T2-weighted sequences in all patients (TR/TE 3,500/120); in four patients axial T2-weighted sequences (TR/TE 3,000/120) were performed.
The flexion MRI imaging protocol of cervical spine consisted for all patients in: sagittal T2-weighted sequences (TR/TE 3,500/120) and axial T2-weighted sequences (TR/TE 3,750/120). Neck-flexion was obtained positioning a head-sponge with an angulation between 25–35 degrees (depending on patient conformation and collaboration).
The section thickness was 4mm, for both sagittal and axial MR imaging in all sequences in neutral and flexed position.
The following diagnostic features were evaluated (Table 1): abnormal cervical curvature, localized cervical cord atrophy in the lower tract (C4–C7), presence of CF, intramedullary signal hyperintensity on T2 weighted sequences, anterior shifting of the posterior wall of the cervical dural sac (ASD) and presence of flow voids (EFV) in the posterior epidural space during flexion.
Full table
Cervical curvature was considered normal when the vertebral bodies C3 through C6 were anterior to a line drawn from C2 through C7. An abnormal, straight or kifotic, curvature was defined when part or all of the vertebral bodies from C3 to C6 met or crossed the line from C2 to C7.
Localized cervical cord atrophy was defined as a decrease in cord size in comparison with the normal cord above and that below the affected level on sagittal and/or axial images (4).
CF was defined as loss of the normal ovoid spinal cord configuration without a narrowed or obliterated adjacent subarachnoid space; a pear-shaped spinal cord was considered as asymmetric CF and a triangular spinal cord was considered as symmetric CF. CF was evaluated on sagittal and axial images; if neutral images were not available, this was assessed on sagittal and axial flexion images.
Intramedullary hyperintesity was considered on axial and/or sagittal T2 images if intramedullary high signal intensity was noted without evidence of other causes of cord compression.
Anterior shifting of posterior dural sac (ASD) and presence of abnormal epidural flow voids (EFV) were evaluated on flexion MRI studies. ASD was defined as an abnormal detachment and anterior dislocation of the posterior wall of dural sac during neck flexion with enlargement of posterior epidural space. EFV, if present, was suggestive of engorgement of posterior epidural venous plexus.
Results
Demographics and clinical features (Table 2)
Full table
Patients were prevalently males (male/female ratio =3/1) with mean age of 17.8 years at the time of clinical presentation. All patients were white and two of them were brothers. Mutations in the glycyl-tRNA synthetase (GARS) gene were excluded in the familial case.
All patients complained of weakness in hand muscles as initial symptoms, associated with hand tremor in three of them and abnormal sweating of the hand palm in two of them. No sensory deficits and weakness at lower limbs were reported by any patients. Distal deep tendon reflexes at upper limbs were absent in all patients with the absence of the right tricipital reflex in one of them. Deep tendon reflexes at lower limbs were normal and no signs of pyramidal tract involvement were present. The clinical involvement at onset was unilateral in six patients (three left-sided and three right-sided) and bilateral asymmetric in two of them, with the right side more affected. With the progression of the disease, all patients but one experienced weakness and wasting of hand muscles and forearm bilaterally, but still asymmetric (with the onset side more affected than the other one). The duration of the progression phase of the disease ranged from eight months (1 patient) to three years (3 patients).
Electrophysiological findings
All patients underwent standard nerve conduction studies (NCS), EMG and motor/sensory evoked potentials (MEP/SEP).
In all patients, NCS and EMG findings were consistent with a spinal metameric disorder involving the C7-T1 myotomes bilaterally (prevalent on the right side in three patients and on the left in two). Sensory conduction and electrophysiologic features at lower limbs were normal. MEP and SEP were normal and we did not observe the disappearance of the spinal potential during the neck flexion in any of the patients.
Cervical MRI findings (Table 3)

Full table
All patients had loss of the normal cervical lordosis (100%) (Figure 1).
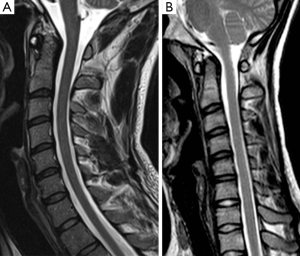
Five patients had loss of attachment and anterior dural shift on flexion MRI with presence of flow voids from venous plexus congestion (62.5%) (Figure 2); three patients had no anterior dislocation of the dural sac and no epidural vein congestion.
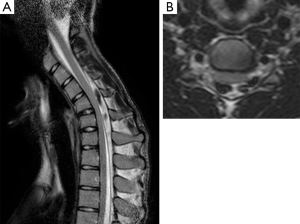
Two patients showed localized cord atrophy, one at C5–C6 and the other at C6–C7 level (25%). Three patients had T2 intramedullary hyperintensities (37.5%) (Figure 3), in both neutral and flexion position.
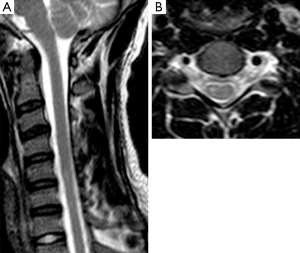
CF was present in 5 patients of 8 (62.5%).
Other findings were: 3 patients presented slight intersomatic disc protrusions: 1 patient between C4 and C5, 2 patients between C6 and C7 and 1 patient at C3–C4 level, present in both neutral and flexion position.
Treatment
Patients received no treatment for their neurological deficits. Some of them were proposed to undergo collar therapy, but they were not compliant and suspended the therapy after few months. One patient underwent surgical treatment with duraplasty. The progression of the disease ranged from eight months to three years.
Discussion
HD is a benign, self-limited, motor neuron disease, characterized by asymmetric weakness and atrophy of one or both distal upper extremities. Its pathogenesis is strongly debated. The main hypothesis is that HD is a myelopathy related to flexion movements of the neck, during which there is a forward displacement of a tight dural sac compressing the cervical cord (18). The compression would lead to an increased intramedullary pressure causing microcirculatory disturbance in the anterior horn. Hirayama et al. speculated that disproportionate growth between the vertebral column and its canal contents during growth spurt, could cause a tight dural sac leading to the flexion myelopathy (1). Strenuous exercise of the arms and repeated neck flexions, were noted in affected patients and speculated as risk factors (5,20).
Nevertheless some studies showed no substantial clinical and radiological modifications with neck flexion and contradict this hypothesis (12,17).
Some authors proposed atopy and elevated IgE serum levels as participating factors, based on the evidence of some cases of atopic myelitis and a higher frequency of allergic diseases and hyperIgEaemia in patients with HD; some patients had also more severe disabilities (21-24). An involvement of intrathecal immune/inflammatory process was proposed by Tanaka et al. (25), that revealed intrathecal upregulation of IFN-gamma and MIP-1beta in HD patients.
Gamez et al. (26) revealed no relationship between HD pathogenesis and SMN1/SMN2 genes, associated with some motor neuron diseases.
From a clinical point of view, HD has a stationary stage after a progressive phase of the neurological deficits interesting the C7, C8 and T1 myotomes of about 1–5 years.
The disease affects young people, predominantly men, in the second to third decades of life and is characterized by the insidious onset of predominantly unilateral or bilateral asymmetric weakness and atrophy of the distal forearm and hand including thenar, hypothenar, interossei muscles and wrist flexors and extensors, with sparing of the brachioradialis muscle. This topography gives the characteristic appearance of “oblique amyotrophy” (1).
The duration of the progressive phase of the disease in our population was between eight months and three years and the presence of weakness and atrophy of forearm and arm was consistent with the literature; in our series, the most common symptom at onset was weakness (50%). All our patients had the classic pattern of “oblique amyotrophy”.
Other typical clinical features include “cold paresis”, worsening of symptoms with cold exposure, and no abnormalities of tendons reflexes, sensory disturbance and pyramidal signs (14,15,27).
Muscular impairment could be initially unilateral, with right upper limb more frequently affected, but secondary asymmetric bilateral involvement is possible (28). Rarely, symptoms could be bilateral and symmetric (1) with a reported prevalence from 3% to 10% (5). In our series, unilateral involvement at onset was present in six patients. Two patients had bilateral involvement, in one case at onset; in both, impairment was asymmetric with right side more affected.
Atrophied muscles can show signs of acute or chronic denervation at EMG and reduction of amplitude of compound muscle action potentials; nevertheless, in patients with unilateral amyotrophy, homonymous muscles of the unaffected side can show denervation. In about 25–50% of cases non-atrophic muscles of the affected side sometimes can show denervation (i.e., triceps brachii, brachioradialis, biceps brachii and deltoid muscles). Motor nerve conduction velocities are usually normal (1,29).
Motor evoked potential after transcranial magnetic stimulation shows an increased latency and decreased amplitude which is temporally aggravated by neck flexion.
During the progressive phase of the disease, neck flexion could lead to a decrease of F-wave persistency; other features are increased latency and high amplitude waveform suggesting denervation/reinnervation. In patients with severe wasting, the F-wave may become unrecordable (27).
Contrariwise, Misra et al. and Ammendola et al. found no significant differences between standard and flexed position for F-wave parameters, suggesting a different etiology for HD than flexion myelopathy (2,12). Hassan et al. and Ghosh et al. showed no electromyographic abnormalities of C5 and C6 myotomes (27,28).
In our series chronic denervation at EMG of C7, C8 and T1 myotomes was evident in all patients; C5 and C6 were spared. Electromyographic abnormalities were bilateral, but some patients had right (3) or left (2) prevalence, in agreement with their symptoms. No sensitive conduction abnormalities were found.
Diagnostic imaging is very important to support clinical suspect of HD. Conventional radiographic examinations of the cervical spine may show loss of cervical lordosis (27) or a hyperflexed cervical motion rate (30). In the 80’s, myelography and CT-myelography, in neutral and neck flexed position, were used to evaluate patients with suspected HD demonstrating atrophy of the lower cervical cord and forward displacement of a tightened dural sac (14). However, myelography is an invasive investigation that needs to retain the contrast medium in the subarachnoid space during neck flexion, so it is hard to perform. On axial images at CT myelography asymmetric CF could be seen, with epidural space appearing as an area of hypodensity behind the dura (31).
MRI is the best diagnostic tool in the diagnosis of HD; it is a non-invasive modality that can reveal some features when performed in neutral and neck-flexion position. MRI can confirm clinical diagnosis and exclude other conditions responsible for the neurological deficits leading to a correct patient management and therapy, limiting arm impairment.
Forward shifting of the posterior dural sac and engorgement of posterior epidural venous plexus are considered the characteristic signs of HD in flexion cervical MRI (1,15,32).
Some authors (31,33,34) reported the presence of forward shifting of the dural sac in all their patients. Nevertheless this sign is not always present in HD patients. Zhou et al. and Lheman et al. reported a prevalence respectively of 71% and 76% (10,35). Interestingly, Lai et al. (36) demonstrated the presence of anterior shifting of posterior dural sac in 46% of healthy subjects, of a lesser degree compared to patients group and without evidence of cord compression. Nonetheless, they suggested that forward shifting of the posterior dural sac on neck flexion can occur as normal variation and its presence does not always lead to diagnosis of HD. In our series anterior displacement of posterior cervical dural sac was present in five of eight patients, a percentage consistent with the literature. Hou et al. (37) postulated that neck flexion angle had effects on the presence of anterior dural shift, evidence of cervical epidural space and flow voids; they suggest a flexion of 25 degrees at least, 35 degrees as the best choice.
In patients with HD an engorged epidural venous plexus was seen in MRI flexion studies; five of eight patients had this sign in our study. These vascular changes were confirmed by angiographic and CT-venography exams (11,38,39) and a possible additional role had been proposed to concur with the arterial ischemia in the pathogenesis of HD. Venous engorgement is suspected to be secondary to some co-existing mechanisms such as an impaired venous drainage toward the jugular veins during neck flexions and an increased flow to posterior internal vertebral venous plexus resulting from the negative pressure in posterior epidural space as a result of anterior shifting of the dura (11,27). Nevertheless, in a case report, Patel et al. (40), showed no change in venous epidural pressure in the flexed position suggesting a passive dilatation of the epidural plexus. Recently, Gupta et al. utilized the 3D-CISS sequence to enhance the presence of the epidural flow voids, because it allows better cerebrospinal fluid to tissue contrast (41). On flexion sagittal T1-weighted images, posterior detachment with forward shifting of the dural sac allows to observe a widened posterior epidural space appearing as a low intensity “crescent shaped epidural mass” with flow voids and uniform enhancement of this crescentic area on post-contrast images (4,6,31,41).
Among the other MRI features, asymmetric CF, localized lower cervical cord atrophy and loss of posterior dural attachment have a reported accuracy of about 80% in HD diagnosis (27,42).
Chen et al. (42) proposed loss of attachment with the subjacent lamina (LOA) as the most reliable sign on neutral position cervical MRI; they reported a prevalence in their series of 93% among the patients group. Because of the rarity of HD in Europe and Italy we did not use standardized MRI protocols and we could not evaluate LOA because of the lack of axial T2-weighted images in neutral position for all patients. So, despite of its rarity, we want to underline the importance of suspecting Hirayama’s disease and establishing standardized MRI protocols to research diagnostic features on both axial and neck-flexed position. We suggest that every patient with a clinical suspect of HD would undergo the following MR C-spine protocol including at least:
Sagittal T1-weighted and T2-weighted sequences and axial T2 or T2*-weighted sequence in neutral position; sagittal T2-weighted sequences and axial T2 or T2*-weighted sequence in a neck- flexion of 35 degrees as best choice, at least 25 degrees (being the axial T2 or T2*-weighted sequence during flexion perpendicular to the spinal cord). Sagittal T1-weighted sequences in neck-flexion before and after gadolinium intravenous administration could be added.
CF has a various prevalence in different case series. Yin et al., Hassan et al. and Raval et al. (6,27,31) reported a 100% of prevalence while Lehman et al. (10) and Yang et al. (32) found it respectively in 48% and 43%. Finsterer et al. (9) executed MRI studies in the neutral position and reported asymmetric CF only in one patient of seven. In our series, CF was present in five patients of eight (62.5%).
Localized cervical cord atrophy was reported by Hirayama and Tokumaru in about 50% of cases (14). Controversal results are present in literature. Sonwalkar et al. and Raval et al. (4,31) depicted cord atrophy in 100% of their patients, Hassan et al. in 82% of cases. Chen et al. demonstrated localized cervical cord atrophy only in their patients group (42). Among our patients, only two presented cervical cord atrophy, similar to results of Ghosh et al. that described it in 33% of cases (28).
Contrariwise to other studies, Boelmans et al. executed neutral position MRI in eight patients and no one of them had CF or localized cord atrophy. Diffusion tensor imaging (DTI) evaluation showed no alteration of the cortico-spinal tract confirming that HD is a primary spinal motor neuron disease (43). Recently, attention was pointed on the presence of a localized cord atrophy as a “sand-watch”—like pattern on sagittal images as a sign of disease (41,44).
All our patients had loss of normal cervical lordosis. In agreement with our finding were the studies of Hassan et al. and Raval et al. that depicted loss of cervical curvature in 91% and 100% of their patients (27,31). Chen et al. in their series founded a statistically significant difference between healthy and patients group, with prevalence of this sign in the latter. Loss of cervical lordosis is thought to be in relationship with the presence of a tight dural sac in patients with HD (42).
Two of our patients (25%) showed T2 hyperintensities of the spinal cord respectively at C5–C6 and C6–C7 levels. High signal alterations on T2 weighted images without cord compression are described in the literature, but this finding is inconstant, presenting in about one third or less of the patients (10,27,34,42).
Syringomyelia, amyotrophic lateral sclerosis, cervical spondilosis associated with myelopathy, spinal cord tumor and traumatic myelopathy, may cause localized amyotrophy of the distal arm, and should be differentiated from HD by imaging modalities. In our familial case we looked for mutations in GARS to rule out distal spinal muscular motor atrophy type V (45).
Collar therapy or surgical intervention had been proposed as treatment for HD. Cervical collar is used to avoid neck movements and to prevent progressive muscular weakness at early stage of the disease with good response; its application for three to four years has been advocated (1,28,46). Surgical treatment with cervical spinal fusion and duraplasty had encouraging results in selected patients. Some authors report better outcomes in patients treated surgically than those treated conservatively (47,48). Particularly in patients that do not improve with collar therapy a surgical intervention could be beneficial, because it gives a permanent stable fixation with a shorter period of immobilization (27,49). Arrese et al. (50) executed in a case duraplasty without spinal fusion to avoid the spinal cord compression without limiting cervical motion; recently also Ito et al. (51) treated successfully six patients with duraplasty without spinal fusion, improving neurological deficits and supporting tight dural canal theory for HD. To all our patients it was suggested to avoid sports or situations that might induce neck trauma. Some of them were proposed to undergo collar therapy, but they were not compliant and suspended the therapy after few months. One patient underwent surgical treatment with duraplasty.
Conclusions
HD is a rare entity, more frequent in Japan and Asian countries, characterized by unilateral or bilateral asymmetric weakness and atrophy of the hands and forearms, particularly in men. It’s a self-limited pathology, but it has to be differentiated early from other diseases that could determinate myelopathy and amyotrophy to establish a correct therapy and limit arms impairment.
MRI is very important to confirm clinical suspect of HD and a standardized MRI protocol using axial and sagittal images in both neutral and flexed position is needed in order to make diagnosis and to follow up affected patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our work is not an experimental protocol; we evaluated retrospectively our patients, so there is not an ethical committee approval. We obtained written informed consent from all of our patients.
References
- Hirayama K. Juvenile muscular atrophy of distal upper extremity (Hirayama disease). Intern Med 2000;39:283-90. [Crossref] [PubMed]
- Misra UK, Kalita J. Central motor conduction in Hirayama disease. Electroencephalogr Clin Neurophysiol 1995;97:73-6. [Crossref] [PubMed]
- Chen CJ, Chen CM, Wu CL, Ro LS, Chen ST, Lee TH. Hirayama disease: MR diagnosis. AJNR Am J Neuroradiol 1998;19:365-8. [PubMed]
- Sonwalkar HA, Shah RS, Khan FK, Gupta AK, Bodhey NK, Vottath S, Purkayastha S. Imaging features in Hirayama disease. Neurol India 2008;56:22-6. [Crossref] [PubMed]
- Tashiro K, Kikuchi S, Itoyama Y, Tokumaru Y, Sobue G, Mukai E, Akiguchi I, Nakashima K, Kira J, Hirayama K. Nationwide survey of juvenile muscular atrophy of distal upper extremity (Hirayama disease) in Japan. Amyotroph Lateral Scler 2006;7:38-45. [Crossref] [PubMed]
- Yin B, Liu L, Geng DY. Features of Hirayama disease on fully flexed position cervical MRI. J Int Med Res 2011;39:222-8. [Crossref] [PubMed]
- Dejobert M, Geffray A, Delpierre C, Chassande B, Larrieu E, Magni C. Hirayama disease: three cases. Diagn Interv Imaging 2013;94:319-23. [Crossref] [PubMed]
- Kang JS, Jochem-Gawehn S, Laufs H, Ferbert A, Vieregge P, Ziemann U. Hirayama disease in Germany: case reports and review of the literature. Nervenarzt 2011;82:1264-72. [Crossref] [PubMed]
- Finsterer J, Löscher W, Wanschitz J, Baumann M, Quasthoff S, Grisold W. Hirayama disease in Austria. Joint Bone Spine 2013;80:503-7. [Crossref] [PubMed]
- Lehman VT, Luetmer PH, Sorenson EJ, Carter RE, Gupta V, Fletcher GP, Hu LS, Kotsenas AL. Cervical spine MR imaging findings of patients with Hirayama disease in North America: a multisite study. AJNR Am J Neuroradiol 2013;34:451-6. [Crossref] [PubMed]
- Ciceri EF, Chiapparini L, Erbetta A, Longhi L, Cicardi B, Milani N, Solero CL, Savoiardo M. Angiographically proven cervical venous engorgement: a possible concurrent cause in the pathophysiology of Hirayama’s myelopathy. Neurol Sci 2010;31:845-8. [Crossref] [PubMed]
- Ammendola A, Gallo A, Iannaccone T, Tedeschi G. Hirayama disease: three cases assessed by F wave, somatosensory and motor evoked potentials and magnetic resonance imaging not supporting flexion myelopathy. Neurol Sci 2008;29:303-11. [Crossref] [PubMed]
- Restuccia D, Rubino M, Valeriani M, Mirabella M, Sabatelli M, Tonali P. Cervical cord dysfunction during neck flexion in Hirayama’s disease. Neurology 2003;60:1980-3. [Crossref] [PubMed]
- Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology 2000;54:1922-6. [Crossref] [PubMed]
- Huang YL, Chen CJ. Hirayama disease. Neuroimaging Clin N Am 2011;21:939-50. ix-x. [Crossref] [PubMed]
- Hirayama K. Juvenile muscular atrophy of distal upper extremity (Hirayama disease): focal cervical ischemic poliomyelopathy. Neuropathology 2000;20 Suppl:S91-4. [Crossref] [PubMed]
- Schröder R, Keller E, Flacke S, Schmidt S, Pohl C, Klockgether T, Schlegel U. MRI findings in Hirayama’s disease: flexion-induced cervical myelopathy or intrinsic motor neuron disease? J Neurol 1999;246:1069-74. [Crossref] [PubMed]
- Kikuchi S, Shinpo K, Niino M, Higashi T, Tashiro K. Cervical myelopathy due to a "tight dural canal in flexion" with a posterior epidural cavity. Intern Med 2002;41:746-8. [Crossref] [PubMed]
- Hirayama K. Non-progressive juvenile spinal muscular atrophy of the distal upper limb (Hirayama’s disease). In: Vinken PJ, Bruyn GW, Klawans HL. editors. Diseases of the Motor System. Handbook of Clinical Neurology. Amsterdam: Elsevier, 1991;59:107-20.
- Biondi A, Dormont D, Weitzner I Jr, Bouche P, Chaine P, Bories J. MR Imaging of the cervical cord in juvenile amyotrophy of distal upper extremity. AJNR Am J Neuroradiol 1989;10:263-8. [PubMed]
- Kira J, Ochi H. Juvenile muscular atrophy of the distal upper limb (Hirayama disease) associated with atopy. J Neurol Neurosurg Psychiatry 2001;70:798-801. [Crossref] [PubMed]
- Ito S, Kuwabara S, Fukutake T, Tokumaru Y, Hattori T. HyperIgEaemia in patients with juvenile muscular atrophy of the distal upper extremity (Hirayama disease). J Neurol Neurosurg Psychiatry 2005;76:132-4. [Crossref] [PubMed]
- Osoegawa M, Ochi H, Mei FJ, Minohara M, Murai H, Taniwaki T, Kira J. Th2 shift in juvenile muscular atrophy of distal upper extremity: a combined allergological and flow cytometric analysis. J Neurol Sci 2005;228:87-92. [Crossref] [PubMed]
- Ochi H, Murai H, Osoegawa M, Minohara M, Inaba S, Kira J. Juvenile muscular atrophy of distal upper extremity associated with airway allergy: two cases successfully treated by plasma exchange. J Neurol Sci 2003;206:109-14. [Crossref] [PubMed]
- Tanaka M, Ishizu T, Ochi H, Kawano Y, Ohyagi Y, Kira J. Intrathecal upregulation of IFN-gamma and MIP-1beta in juvenile muscular atrophy of the distal upper extremity. J Neurol Sci 2008;275:74-7. [Crossref] [PubMed]
- Gamez J, Also E, Alias L, Corbera-Bellalta M, Barceló MJ, Centeno M, Raguer N, Gratacós M, Baiget M, Tizzano EF. Investigation of the role of SMN1 and SMN2 haploinsufficiency as a risk factor for Hirayama’s disease: clinical, neurophysiological and genetic characteristics in a Spanish series of 13 patients. Clin Neurol Neurosurg 2007;109:844-8. [Crossref] [PubMed]
- Hassan KM, Sahni H. Nosology of juvenile muscular atrophy of distal upper extremity: from monomelic amyotrophy to Hirayama disease--Indian perspective. Biomed Res Int 2013;2013:478516.
- Ghosh PS, Moodley M, Friedman NR, Rothner AD, Ghosh D. Hirayama disease in children from North America. J Child Neurol 2011;26:1542-7. [Crossref] [PubMed]
- Jin X, Jiang JY, Lu FZ, Xia XL, Wang LX, Zheng CJ. Electrophysiological differences between Hirayama disease, amyotrophic lateral sclerosis and cervical spondylotic amyotrophy. BMC Musculoskelet Disord 2014;15:349. [Crossref] [PubMed]
- Xu X, Han H, Gao H, Hou C, Fan D, Fu Y, Sun Y. The increased range of cervical flexed motion detected by radiographs in Hirayama disease. Eur J Radiol 2011;78:82-6. [Crossref] [PubMed]
- Raval M, Kumari R, Dung AA, Guglani B, Gupta N, Gupta R. MRI findings in Hirayama disease. Indian J Radiol Imaging 2010;20:245-9. [Crossref] [PubMed]
- Yang G, Yang X, Zhang M, Yang Y, Xiao B, Li G, Yang D, Wang X. Hirayama disease in children from mainland of China. J Child Neurol 2014;29:509-13. [Crossref] [PubMed]
- Pradhan S, Gupta RK. Magnetic resonance imaging in juvenile asymmetric segmental spinal muscular atrophy. J Neurol Sci 1997;146:133-8. [Crossref] [PubMed]
- Fu Y, Pei X, Zhang J, Kang D, Han H, Fan D. Morphological changes of the lower cervical spinal cord under neutral and fully flexed position by MRI in Chinese patients with Hirayama’s disease. Amyotroph Lateral Scler 2008;9:156-62. [Crossref] [PubMed]
- Zhou B, Chen L, Fan D, Zhou D. Clinical features of Hirayama disease in mainland China. Amyotroph Lateral Scler 2010;11:133-9. [Crossref] [PubMed]
- Lai V, Wong YC, Poon WL, Yuen MK, Fu YP, Wong OW. Forward shifting of posterior dural sac during flexion cervical magnetic resonance imaging in Hirayama disease: an initial study on normal subjects compared to patients with Hirayama disease. Eur J Radiol 2011;80:724-8. [Crossref] [PubMed]
- Hou C, Han H, Yang X, Xu X, Gao H, Fan D, Fu Y, Sun Y, Liu B. How does the neck flexion affect the cervical MRI features of Hirayama disease? Neurol Sci 2012;33:1101-5. [Crossref] [PubMed]
- Elsheikh B, Kissel JT, Christoforidis G, Wicklund M, Kehagias DT, Chiocca EA, Mendell JR. Spinal angiography and epidural venography in juvenile muscular atrophy of the distal arm "Hirayama disease". Muscle Nerve 2009;40:206-12. [Crossref] [PubMed]
- Waung MW, Grossman AW, Barmada SJ, Josephson SA, Dillon WP, Ralph JW. Pearls & oy-sters: the use of CT venography in Hirayama disease. Neurology 2012;79:e38-40. [Crossref] [PubMed]
- Patel TR, Chiocca EA, Freimer ML, Christoforidis GA. Lack of epidural pressure change with neck flexion in a patient with Hirayama disease: case report. Neurosurgery 2009;64:E1196-7; discussion E1197.
- Gupta K, Sood S, Modi J, Gupta R. Imaging in Hirayama disease. J Neurosci Rural Pract 2016;7:164-7. [Crossref] [PubMed]
- Chen CJ, Hsu HL, Tseng YC, Lyu RK, Chen CM, Huang YC, Wang LJ, Wong YC, See LC. Hirayama flexion myelopathy: neutral-position MR imaging findings--importance of loss of attachment. Radiology 2004;231:39-44. [Crossref] [PubMed]
- Boelmans K, Kaufmann J, Schmelzer S, Vielhaber S, Kornhuber M, Münchau A, Zierz S, Gaul C. Hirayama disease is a pure spinal motor neuron disorder--a combined DTI and transcranial magnetic stimulation study. J Neurol 2013;260:540-8. [Crossref] [PubMed]
- Cortese R, Simone IL. The role of imaging in Hirayama disease. J Neurosci Rural Pract 2016;7:5-6. [Crossref] [PubMed]
- Blumen SC, Drory VE, Sadeh M, El-Ad B, Soimu U, Groozman GB, Bouchard JP, Goldfarb LG. Mutational analysis of glycyl-tRNA synthetase (GARS) gene in Hirayama disease. Amyotroph Lateral Scler 2010;11:237-9. [Crossref] [PubMed]
- Tokumaru Y, Hirayama K. Cervical collar therapy for juvenile muscular atrophy of distal upper extremity (Hirayama disease): results from 38 cases. Rinsho Shinkeigaku 2001;41:173-8. [PubMed]
- Konno S, Goto S, Murakami M, Mochizuki M, Motegi H, Moriya H. Juvenile amyotrophy of the distal upper extremity: pathologic findings of the dura mater and surgical management. Spine (Phila Pa 1976) 1997;22:486-92. [Crossref] [PubMed]
- Lin MS, Kung WM, Chiu WT, Lyu RK, Chen CJ, Chen TY. Hirayama disease. J Neurosurg Spine 2010;12:629-34. [Crossref] [PubMed]
- Imamura H, Matsumoto S, Hayase M, Oda Y, Kikuchi H, Takano M. A case of Hirayama’s disease successfully treated by anterior cervical decompression and fusion. No To Shinkei 2001;53:1033-8. [PubMed]
- Arrese I, Rivas JJ, Esteban J, Ramos A, Lobato RD. A case of Hirayama disease treated with laminectomy and duraplasty without spinal fusion. Neurocirugia (Astur) 2009;20:555-8; discussion 558. [Crossref] [PubMed]
- Ito H, Takai K, Taniguchi M. Cervical duraplasty with tenting sutures via laminoplasty for cervical flexion myelopathy in patients with Hirayama disease: successful decompression of a "tight dural canal in flexion" without spinal fusion. J Neurosurg Spine 2014;21:743-52. [Crossref] [PubMed]


