
Neuroradiology of acute pathologies in adults with hematologic malignancies: a pictorial review
Introduction
Hematologic malignancies (HM) include a wide and heterogeneous array of disorders. They can be classified according to the clinical findings, morphology, immunophenotype and genetic features of cancer cells as defined by the 4th edition of the World Health Organization Classification of Tumors of Hematopoietic and Lymphoid tissues (1).
More commonly, these entities are grouped based on their tissue of origin:
- Lymphomas: neoplasms arising from lymphocyte precursors or mature lymphocytes, presenting as discrete tumoral masses with nodal or extra-nodal involvement. Clinical manifestations are caused by tumor growth and/or systemic changes (2).
- Plasma cell neoplasms: neoplasms arising from mature B lymphocytes. These include multiple myeloma (MM), plasmacytoma and Waldenstrom macroglobulinemia (WM) (3). More specifically, MM is characterized by an uncontrolled proliferation of plasma cells in the bone marrow with production of monoclonal immunoglobulin, detectable in the serum and/or urine.
- Leukemias: neoplastic proliferation of hematopoietic cells with a typical involvement of bone marrow and often presenting with a high number of malignant cells in the blood and, rarely, growth of solid tumoral masses. The most frequent clinical manifestations are anemia, immunosuppression, or coagulation disorders, depending on the altered status of hematopoietic function (2).
Nervous system involvement in patients affected by HM is a serious complication that deserves rapid diagnosis and treatment, because it could lead to acute clinical worsening with or without lethal outcome as well as chronic sequelae.
The nervous system can be involved directly by proliferation of tumor cells or, indirectly, as a result of systemic malfunction induced by HM. Furthermore, the nervous system is also exposed to treatment-related toxicity by chemotherapeutic drugs, radiation therapy and/or bone marrow transplantation (2,4).
Neurological signs and/or symptoms related to the above-mentioned disorders often present with sudden onset and require prompt and correct diagnosis driven by clinical status, laboratory data and imaging techniques such as computed tomography (CT) as first imaging, and magnetic resonance imaging (MRI) in unclear cases (5).
Neuroimaging plays an essential role in solving clinical questions and promptly guide the correct treatment when HM affects the nervous system. Non-contrast brain CT is usually the first imaging modality when patients with HM present with a focal neurological deficit. Depending on the clinical scenario and associated findings, it may be followed by a contrast-enhanced study including CT angiography and CT perfusion. Although CT remains the most accessible and fast technique, magnetic resonance (MR), due to its superior sensitivity in investigating brain parenchyma and meninges, is the gold standard in imaging. Contrast-enhanced MR and diffusion-weighted imaging (DWI) are usually part of the standard protocol and extremely important to better define the spatial location and suggest the tissue nature. Additionally, MR is the best technique to follow-up and monitor treatment response (2,6,7).
The purpose of this pictorial review is to illustrate and discuss imaging findings in common and uncommon acute disorders involving the nervous system in adults with HM, with discussion of findings that should be kept in mind by radiologists facing these entities.
HM
The most common HM which can involve central nervous system (CNS), related imaging findings, and major research studies included are summarized in Tables 1-3 (11).
Table 1
| Disease | Location | CT | MRI |
|---|---|---|---|
| Primary CNS lymphoma (8-10) | Parenchymal lesion, solitary or multiple (40%) Common: - Periventricular (50%) and superficial white matter in the supratentorial brain Less common: - Corpus callosum and thalamus/basal ganglia - Cerebellum, brainstem, spinal cord and leptomeninges (less common) |
Unenhanced: - Hypo- or isodense parenchymal lesions Post-contrast: - Homogeneous moderate-marked enhancement - Heterogeneous and irregular “ring-like” (necrosis) Moderate vasogenic edema |
T1: - Hypo- or isointense in relation to gray matter T2/FLAIR: - Hypo-intense (rarely as hyperintensities with no enhancement on post contrast) in relation to gray matter DWI: - Markedly restricted diffusion with low ADC (more than in high-grade gliomas or metastases) GRE T2*/SWI: - Not signs of hemorrhage or calcifications T1 C+ (Gd): - Homogeneous moderate-marked enhancement - Heterogeneous and irregular “ring-like” (necrosis) - Linear enhancement along Virchow-Robin perivascular spaces is considered highly suggestive of PCNSL Moderate vasogenic edema |
| Secondary CNS lymphoma (2,7,11) | Parenchymal (25%): - Paraventricular or superficial Leptomeningeal (75%) Sub-ependymal Dural Cranial nerve |
Communicating hydrocephalus Post-contrast: - Enhancing lesions |
T1 C+ (Gd): - Enhancing lesions Communicating hydrocephalus |
| MM (12-14) | Leptomeningeal disease (60%) Cranial nerve Dural disease or direct extension into the CNS from bone (skull or vertebral myelomatous lesions penetrating at least to the subdural space) Intraparenchymal lesions |
Diffusely thickened linear or nodular pachymeningeal Single or multiple masses Post-contrast: - Enhancing lesions |
T1: - Hypointense or isointense, to bone marrow, in case of diffuse involvement T2 with fat-suppression: - Hyperintense DWI/ADC: - Mildly restricted diffusion (high signal on high b-value - DWI compared to the very low signal of normal background marrow) T1 C+ (Gd): - Homogeneous enhancement |
| Leukemia (2,15) | Leptomeningeal Cranial nerve Dura-based focal masses |
Unenhanced: - Iso/hyperattenuating lesions - Thickening of leptomeninges, nerve root and optic nerve Post-contrast: - Enhancing lesions |
T2/FLAIR: - Hyperintense compared with white matter DWI/ADC: - Restricted diffusion T1 C+ (Gd): - Homogeneous enhancement |
HM, hematologic malignancies; CT, computed tomography; MRI, magnetic resonance imaging; CNS, central nervous system; T1, T1-weighted; T2, T2-weighted; FLAIR, fluid-attenuated inversion recovery; DWI, diffusion weighted imaging; ADC, apparent diffusion coefficient; GRE T2*, gradient echo T2*; SWI, susceptibility-weighted imaging; T1 C+ (Gd), post-contrast T1-weighted; PCNSL, primary CNS lymphoma; MM, multiple myeloma.
Table 2
| Category | Etiology and location | Imaging findings |
|---|---|---|
| Bacterial (16,17) | ||
| Meningitis | Streptococcus pneumoniae Neisseria meningitidis Listeria monocytogenes (immunocompromised patients) TB Pseudomonas aeruginosa (neurosurgery patients) |
Subarachnoid spaces -T1: isointense -T2/FLAIR: hyperintense with cerebral edema -DWI/ADC: restricted diffusion -T1 C+ (Gd): leptomeningeal enhancement -Hydrocephalus |
| Abscess | Nocardiosis TB |
Round/ovoid lesion -T1: hypointense -T2/FLAIR: hyperintense, with perilesional vasogenic edema -DWI/ADC: homogenous diffusion restriction of the necrotic core -GRE T2*/SWI: dual rim appearance of the capsule (peripheric hypointensity and relatively hyperintense inner rim) -T1 C+ (Gd): peripheral rim enhancement |
| Parasitic (16,17) | Toxoplasma gondii (ALLO-HSCT patients, after organ transplantation or ingestion of contaminated food) Location: - Basal ganglia and thalami - Corticomedullary junction and posterior fossa |
Multifocal mass lesions -T1: hypointense -T2/FLAIR: hyperintense or laminated, with perilesional vasogenic edema -DWI/ADC: peripheral diffusion restriction -GRE T2*/SWI: peripheral hemorrhage occasionally -T1 C+ (Gd): rim enhancement, with internal eccentric mural nodule (“eccentric target sign”) |
| Viral (17,18) | ||
| Encephalitis | HSV-1 (most common in adults) Location: - Bilateral asymmetric cortical and sub-cortical - Anterior and medial temporal lobes, insular cortex, cingulate gyri - Widespread cortical involvement, brainstem, or posterior fossa, in immunocompromised patients |
T1: patchy regions of hyperintensity as petechial hemorrhages T2/FLAIR: hyperintensity of affected white matter and cortex GRE T2*/SWI: hypointensity with “blooming” DWI/ADC: early: patchy cortical/subcortical diffusion restriction T1 C+ (Gd): sub-acute: gyriform enhancement |
| HHV-6 (post stem cells transplantation) Location: - Limbic encephalitis - Mesial temporal lobes, sparing parahyppocampal gyri |
T1: absent hemorrhage DWI/ADC: diffusion restriction T1 C+ (Gd): absent enhancement |
|
| EBV Location: - Mesial temporal lobe and subcortical white matter |
Non-specific findings T2/FLAIR: hyperintensities |
|
| Ependymitis, retinitis, encephalitis | CMV (in immunosuppressed patients) Location: - Ependyma - Periventricular regions - Retina |
Retinitis, ependymitis-T1: hypointensity peri-ventricular -T2/FLAIR: hyperintensity peri-ventricular -DWI/ADC: diffusion restriction of affected ependyma -T1 C+ (Gd): nodular thickening enhancement Diffuse encephalitis -T2/FLAIR: widespread patchy hyperintense lesions -T1 C+ (Gd): space-occupying lesion with rim-enhancement and surrounding vasogenic edema |
| PML (19-21) | JC polyomavirus (in HIV patients) Frequently fatal No effective therapy Location: - Subcortical white matter of the - Involvement of the subcortical U-fibers with scalloped appearance - Parietal, occipital and frontal lobes, or cerebellar peduncles - Distribution bilateral and asymmetric |
T1: hypointensity T2/FLAIR: patchy regions of hyperintensity DWI/ADC: acute: diffusion restriction peripheral at the edge demyelination T1 C+ (Gd): absent, faint or present in the context of immune reconstitution inflammatory syndrome |
| Fungal (16,17) | ||
| Abscess, meningitis, ischemic stroke or hemorrhage | Aspergillus Location: - Frontal lobes Angio-invasive Bone destructive |
T2/FLAIR: central hyperintensity and peripheral hypo/isointensity (hemorrhagic foci “target” pattern), due to blood breakdown products or iron products GRE T2*/SWI: focal microhemorrhages T1 C+ (Gd): rim-enhancing lesions (abscess); solid enhancing lesions (aspergilloma) |
HM, hematologic malignancies; TB, tuberculosis; T1, T1-weighted; T2, T2-weighted; FLAIR, fluid-attenuated inversion recovery; DWI, diffusion weighted imaging; ADC, apparent diffusion coefficient; T1 C+ (Gd), post-contrast T1-weighted; GRE T2*, gradient echo T2*; SWI, susceptibility-weighted imaging; ALLO-HSCT, allogeneic hematopoietic stem cell transplant; HSV, herpes simplex virus; HHV, human herpesvirus; EBV, Epstein-Barr virus; CMV, cytomegalovirus; PML, progressive multifocal leukoencephalopathy; JC, John Cunningham; HIV, human immunodeficiency virus.
Table 3
| Authors, year | Country | Aim/rationale | Patients | Main conclusions |
|---|---|---|---|---|
| Wang et al., 2021 (22) | Shanghai, China | To assess the value of CT findings in predicting survival of patients with pulmonary B-cell NHL | 84 | Halo sign and pleural involvement are independent prognostic factors for OS |
| Number of lung lesions, cross-lobe sign, and pleural involvement are correlated with PFS | ||||
| Peters et al., 2012 (23) | Kiel, Germany | To prove diagnostic benefit of SWI | 15 | Better differential diagnosis between glioblastomas and lymphomas |
| Radbruch et al., 2013 (8) |
Heidelberg, Germany | To evaluate appearance of ITSS on SWI to differentiate glioblastoma and B-cell PCNSL | 132 | Better differential diagnosis evaluating ITSS of glioblastoma and B-cell PCNSL |
| Kawase et al., 2011 (24) | Kagawa, Japan | To compare uptake of MET and FDG in patients with CNS lymphoma | 13 | No significant difference between T/N ratios using MET-PET and FDG PET |
| Uptake of MET in CNS lymphoma was significantly lower than that of FDG | ||||
| MET PET demonstrated equal sensitivity to FDG PET for primary lesions in CNS lymphoma | ||||
| Puranik et al., 2019 (25) | Mumbai, India | To assess the utility of FET-PET in differentiating between high-grade brain lesions (i.e., high grade gliomas, metastases, PCNSL) | 27 | Good results of FET-PET in differentiating high-grade glial tumors from other high-grade brain lesions when the MRI features are equivocal |
| Schmitz et al., 2016 (26) | Vancouver, Canada | To develop and validate a risk score for relapse in the CNS in patients with DLBCL | 2,164 | CNS-IPI is a highly reproducible tool to estimate the risk of CNS relapse/progression in patients with DLBCL treated with R-CHOP (i.e., rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy |
| Sun et al., 2021 (27) | Zhengzhou, China | To assess quantification of tumor burden in MM patients using whole-body MRI and to find prognostic biomarkers | 95 | Quantitative whole-body functional MRI examination may be used as an effective complement for diagnosis and evaluate tumor burden in MM |
| Ji et al., 2021 (28) | Tianjin, China | To investigate correlation between ADC values from whole-body DWI and water/fat MRI signals from T1-weighted water-fat separation in evaluating bone marrow infiltration in MM | 35 | ADC value combined with water-fat separation parameters could be used for evaluating thoracolumbar bone marrow infiltration in MM |
| All parameters correlated with the proportion of BMPC | ||||
| Shi et al., 2010 (29) | Shanghai, China | To evaluate clinical usefulness of a classification scheme of CES based on symptoms, imaging signs and electrophysiological findings | 39 | Electrophysiological abnormalities and reduced saddle sensation: indices of early diagnosis. Bilateral radiculopathy or sciatica: early stages and indicate a high risk of development of advanced CES |
| Preclinical and early stages have better functional recovery than patients in later stages after surgical decompression | ||||
| Eden et al., 2016 (30) | Birmingham, UK | To evaluate cerebral thrombotic complications associated with L-asparaginase treatment for ALL | 10 | Patients with ALL in treatment with L-asparginase have higher risk of cerebral thrombotic complications |
| Tan et al., 2009 (19) | Baltimore, USA | To describe types of PML in relation to IRIS and the effects of steroid use | 54 | Immune reconstitution following combined antiretroviral therapy may lead to activation of an inflammatory response to detectable or latent JC virus infection |
| Early and prolonged treatment with steroids may be useful | ||||
| Gust et al., 2017 (31) | Washington, USA | To identify risk factors for neurotoxicity in patients in treatment with after CD19 CAR-T cells | 133 | Endothelial dysfunction, increased BBB permeability, high serum concentrations of IL-6 and MCP-1 within 24 hours of CAR-T cell infusion are present in neurotoxicity |
| Masch et al., 2016 (32) | Michigan, USA | To compare differences in DTI and DSC MR perfusion imaging characteristics of recurrent neoplasm and radiation necrosis in brain tumors previously treated with radiotherapy with or without surgery and chemotherapy | 22 | Combined assessment of DTI and DSC MR perfusion properties of new contrast-enhancing lesions is helpful in distinguishing recurrent neoplasm from radiation necrosis |
CT, computed tomography; NHL, non-Hodgkin lymphoma; OS, overall survival; PFS, progression-free survival; SWI, susceptibility-weighted imaging; ITSS, intratumoral susceptibility signals; PCNSL, primary central nervous system lymphoma; MET, 11-C methionine; FDG, fluorodeoxyglucose; CNS, central nervous system; PET, positron emission tomography; FET, 18-fluoride-fluoro-ethyl-tyrosine; MRI, magnetic resonance imaging; DLBCL, diffuse large B-cell lymphoma; IPI, international prognostic index; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone; MM, multiple myeloma; ADC, apparent diffusion coefficient; BMPC, bone marrow plasma-cells; CES, cauda equina syndrome; ALL, acute lymphoblastic leukemia; PML, progressive multifocal leukoencephalopathy; IRIS, Immune reconstitution inflammatory syndrome; JC, John Cunningham; BBB, blood-brain barrier; DTI, diffusion tensor imaging; DSC, dynamic susceptibility contrast; MR, magnetic resonance.
Primary CNS lymphoma (PCNSL)
PCNSL is an extra-nodal form of lymphoma, confined to the cranio-spinal axis without systemic involvement. It accounts for approximately 5% of all non-Hodgkin lymphomas (NHLs), whereas the isolated involvement of CNS by Hodgkin lymphoma is extremely rare. The most prevalent histologic subtype is diffuse large B-cell lymphoma (90%), followed by high-grade Burkitt-like B-cell lymphoma (10%) (7,33). The main risk factor is congenital or acquired immunodeficiency. In fact, PCNSL corresponds to one of the acquired immunodeficiency syndrome (AIDS)-defining malignancies, accounting for about 20% of all NHL cases in AIDS patients (8,9).
Patients can present with general symptoms such as weight loss, nights sweats and fever, but also signs of cerebral and cerebellar involvement, like focal neurologic deficit, seizures, headache, increased intracranial pressure with headache and nausea. In case of spinal involvement symptoms could include back pain and sensory or motor alterations (34). Cerebrospinal fluid examination is the most sensitive technique to evaluate leptomeningeal disease. CT, fluorodeoxyglucose-positron emission tomography (FDG-PET), and mostly MRI contribute to the imaging investigation in order to establish the diagnosis, prognosis, and assessing response to treatments. In fact, MRI is the imaging modality most widely used for treatment response evaluation in patients with CNS lymphoma (7-9,11).
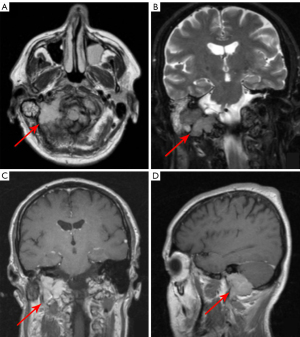
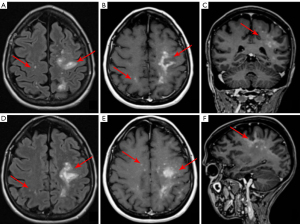
PCNSL appears as solitary or multiple parenchymal lesions with surrounding vasogenic edema, occurring more commonly in immunodeficient patients. Usually, PCNSL has a predilection for the periventricular (50%) and superficial white matter in the supratentorial brain (Figure 1), but also for the corpus callosum, thalamus and basal ganglia. Brain lesions of PCNSL are often located either superficial subpial or deep subependymal.
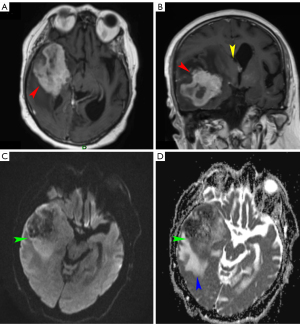
In PCNSL less common sites are the cerebellum, brainstem, spinal cord, leptomeninges, and rarely ventricles (9,22,34). Unenhanced CT typically demonstrates iso- or slightly hyperdense parenchymal lesions with respect to the cortex. MRI shows lesions as hypo- or isointense on unenhanced T1-weighted images, with a variable degree of hypointensity on T2-weighted images with respect to gray matter, with surrounding vasogenic edema. Rarely PCNSL can present as an isolated white matter hyperintensity on T2-weighted sequences with no enhancement on post-contrast T1-weighted sequences (33).
A characteristic feature of PCNSL is true restricted diffusion with low apparent diffusion coefficient values on DWI, due to tumor hypercellularity. This feature is often more pronounced in PCNSL than in high-grade gliomas or metastases (7,8,11).
Susceptibility-weighted imaging (SWI) sequences have been proposed as important MR tool possibly helping to differentiate between glioblastomas and lymphomas, that should be added to the clinical MR tumor protocol (23). SWI usually do not show signs of hemorrhage or calcifications, which are less common findings in PCNSL. However, intratumoral susceptibility signals were found within the T-cell PCNSL, suggesting this feature to be more common for this kind of PCNSL (8).
Post-contrast CT and MRI studies, typically show homogeneous moderate to marked enhancement, despite heterogeneous and irregular “ring-like” enhancement, can sometimes be seen too, due to central necrosis, especially in immunocompromised patients (7,10).
FDG PET/CT, but also methionine PET, have been found useful in staging, prognosis, therapeutic monitoring, but also in the detection of occult systemic lymphoma in patients presenting with CNS involvement. On this respect, no differences in sensitivity (i.e., 100%) were found between tumor-to-contralateral normal brain tissue ratios between 11C-methionine (MET) PET and 2-deoxy-2-[18F] FDG PET in assessing PCNSL, despite the uptake of MET being significantly lower (24).
PCNSL usually presents greater metabolic activity than high-grade gliomas or metastases. Interestingly, the utility of 18-fluoride-fluoro-ethyl-tyrosine positron emission tomography (FET-PET) in discriminating high-grade brain lesions with overlapping MR features among glioma, metastases, and PCNSL has been tested (25). Authors evaluated tumor-to-contralateral white mater ratio, applied receiver operating characteristic curve analysis, and estimated a cutoff of 1.9 to differentiate between tumor of glial or non-glial origin (sensitivity =93.8%; specificity =91%) (24,25,35).
Regarding spatial location, differential diagnosis of lesions involving the corpus callosum and periventricular white matter is wide, including glioma, metastases, toxoplasmosis, tuberculosis, but also cerebral vasculitis and multiple sclerosis.
Treatment of PCNSL varies according to age, comorbidities, and performance status of the patients. There are different options based on high dose multidrug chemotherapy that the penetrate blood-brain barrier (e.g., methotrexate, cytarabine), monoclonal antibody therapy (e.g., rituximab) and whole brain irradiation, with corticosteroids and osmotic agents to reduce vasogenic edema (9,10,23,36). In case of Burkitt lymphoma, extra-nodal involvement is frequent with bone marrow invasion in 70% and leptomeningeal in 40% of adults at the time of diagnosis. The potential rapid progression and the poor prognosis make of paramount importance early diagnosis and treatment with high dose systemic or intra-thecal therapy (35).
Secondary CNS lymphoma
It is more common than the primary form, typically originating as systemic diffusion in 10–15% of patients with aggressive subtypes of NHL, such as diffuse large B-cell lymphoma, Burkitt lymphoma, mantle cells lymphoma. Additionally, secondary CNS lymphoma often presents as relapsed disease (80%) (2,7,11,35).
Depending on the location of the primary site, the risk for CNS involvement is different. Primary orbital, paranasal sinus and testis lymphomas show a higher risk for CNS dissemination, together with elevated levels of lactate dehydrogenase, involvement or more than one extra-nodal site, and advanced disease (i.e., stage III–IV) (26).
Patients can show symptoms and signs arising from multiple sites in the neuroaxis, such as cranial or spinal neuropathies, but also headache, often determined by the increased intracranial pressure due to obstruction of cerebrospinal fluid (CSF) flow/absorption (40%). The most common form of CNS involvement in secondary lymphoma leptomeningeal disease (75%), which can determine an involvement of cranial nerves, spinal cord, and nerve roots. CT is less sensitive than contrast-enhanced MRI, which is the gold standard for the detection of leptomeningeal disease. Other findings are parenchymal enhancing lesions with paraventricular or superficial location, similar to PCNSL, or leptomeningeal, sub-ependymal, dural, and cranial nerve enhancement. Associated obstructive hydrocephalus can be present (2,9,11).
Standard combination therapy used in systemic lymphoma is not effective for secondary CNS lymphoma. Instead, patients are treated with methotrexate with or without radiation therapy (2).
Angiocentric intravascular lymphoma
Angiocentric intravascular lymphoma is a rare subtype of CNS lymphoma, histologically characterized by proliferation of large malignant lymphocytes cells, located in the lumen of small blood vessels of the brain, often determining multifocal ischemic lesions. It may present with neurologic symptoms, such as altered consciousness, sensory or motor deficits, or progressive dementia. CT and MRI manifestations are suggestive for small vessels related to ischemia or demyelination. Patients have a poor prognosis, partly due to a challenging and delayed diagnosis. Treatments is the same as in aggressive NHL (37).
Plasma cell neoplasms
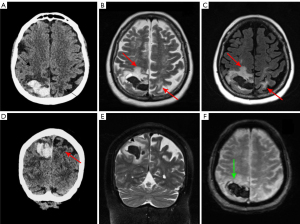
MM represents 15% of HM and it is the most common primary malignant bone malignancy in adults. MM hallmarks are neoplastic proliferation of plasma cells in the bone marrow producing monoclonal immunoglobulins. The most common symptom is bone-related pain, caused by lytic lesions, often leading to pathologic fractures. Systemic manifestations are also present, such as, fatigue, weakness, anemia, and renal failure (12,13). The diagnosis is based on serum and urine electrophoresis to quantify M protein, counts of plasma cells in the bone marrow, and a skeletal survey by low-dose CT, MRI or PET scan. Different variants of MM include classical MM, plasma cell leukemia, and solitary plasmacytoma which can be intra- or extra-medullar solitary tumor, without systemic involvement (Figure 2). Skull and vertebrae are the most common bone sites of involvement (36).
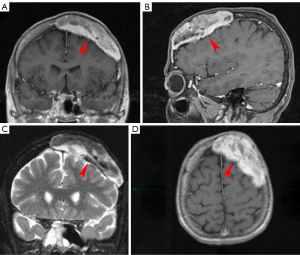
The most typical CNS involvement is diffuse or localized leptomeningeal enhancement (60%). Other manifestations are extra-axial dura-based masses, solitary or multiple intraparenchymal lesions and cranial nerve enhancement. In the spinal canal MM usually presents as an epidural soft-tissue mass, contiguous or non-contiguous with bone. Between HM, MM has the highest risk of spinal cord compression (SCC) (8%) of all form of HM, usually caused by vertebral collapse, an infiltrating paravertebral mass or leptomeningeal involvement. Symptoms include pain (80–90% of patients), acute sensory-motor impairment at various levels and/or autonomic dysfunction (38).
CT without contrast shows osteolytic bone involvement, fractures, and spinal narrowing. To better evaluate spinal cord involvement, including myelopathy and compression MRI is the imaging technique of choice (38).
Epidural soft tissue lesions of MM usually appear hypointense to isointense on T1-weighted images compared to skeletal muscle, isointense to hyperintense on T2-weighted images, and show homogeneous enhancement (27,28).
The goals of treatments are to maintain neurological function, control pain and prevent myelopathy. Initial therapy for MM includes steroids in association with proteasome inhibitors (e.g., bortezomib, carfilzomib) and/or immunomodulant (e.g., thalidomide, lenalidomide) drug and monoclonal antibody (e.g., daratumumab), with or without autologous stem cell transplantation based on age and performance status of the patients. Besides, in patients with SCCs or lytic lesions of the axial skeleton, radiation therapy, bisphosphonate, and percutaneous vertebroplasty could be useful to prevent vertebral collapse leading to serious complications. Radiation therapy is also an option for treatment of plasmacytoma (39).
Leukemia
Leukemia is a term that indicates a group of hematopoietic malignancies originating from myeloid and lymphoid cell lineage, involving the bone marrow, and typically causing anemia, bleedings, and infections. CNS leukemia is caused by an hematogenous spread with a leptomeningeal dissemination, or, indirectly, due to extension from cranial bone involvement (2).
CNS involvement can be observed in acute lymphoblastic leukemia (ALL) because leukemic cells might invade meninges through arachnoid veins with subsequent CNS involvement. Leukemic meningitis can affect the cerebral hemispheres, cranial nerves and more often, the optic nerve or spinal cord. Common symptoms are headache and changed mental status (30,40).
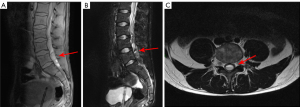
Mass lesions are uncommon in leukemia, however, occasionally chloromas can be detected as solid tumors composed of myeloid leukemic blasts cells adjacent to the skull or facial bones often with a dural attachment. They can be also found in other non-CNS anatomical sites, and their presence is usually associated with aggressive systemic leukemia (41).
Diagnosis is achieved by cerebral spinal fluid examination, while MRI can be helpful for detection and extension evaluation. MR findings in nervous system leukemia include leptomeningeal enhancement, thickening and abnormal enhancement of nerve roots and/or optic nerves, and dural-based focal masses, possibly appearing as iso/hyperattenuating on unenhanced CT (Figure 3). MR shows T2-hyperintense lesions compared with white matter, with restricted diffusion. At post-contrast CT or MRI these lesions usually present marked and homogeneous enhancement (40).
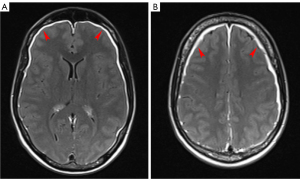
Treatment of choice is with chemotherapy, and/or intrathecal chemotherapy (41).
Etiopathogenetic classification
According to the underlying etiopathogenetic mechanism, we propose a classification of the acute conditions involving the nervous system in adult patients affected by HM. These can be grouped into three categories: tumoral mass effect, vascular and immune-related.
Tumoral mass effect
Mass-forming HM with potential nervous system involvement usually show compressive and/or infiltrative behavior, with slowly progressive or fast growth (42). Acute clinical onset with focal or generalized neurological symptoms including headache, focal deficit, pain, motor or sensory impairment, cranial nerves palsies, changes in mental status, seizures, and coma (Figures 1,2,4,5).
Elevated intracranial pressure (EIP)
EIP needs to be managed rapidly, because it could lead to irreversible neurological deficit or even death. Any rise in the volume content of the non-expandable calvarium will cause increased intracranial pressure. EIP can have various causes. It is mainly provoked by a mass lesion (e.g., brain tumor, abscess), by obstructive hydrocephalus due to leptomeningeal disease, by obstruction of venous outflow due to venous thrombosis, or also as a consequence of vasogenic edema, accompanying primary and secondary tumors, or cytotoxic edema, in cases of stroke or meningoencephalitis (43). As a result of an increased pressure gradient, the main severe sequelae of EIP are decreased cerebral decreased blood flow with possible secondary ischemia and herniation of brain structures into adjacent compartments. The first non-specific symptom is usually headache, but it can present with many other symptoms including nausea, vomiting and diplopia. In more advanced stages, involvement of the brainstem can be associated with Cushing’s triad (i.e., hypertension, bradycardia, and irregular breathing). CT and MRI can reveal signs of increased EIP such as enlarged ventricles, volume reduction of subarachnoid spaces and brain herniation, but also the underlying causes such as tumors, abscesses, and hematomas (44,45).
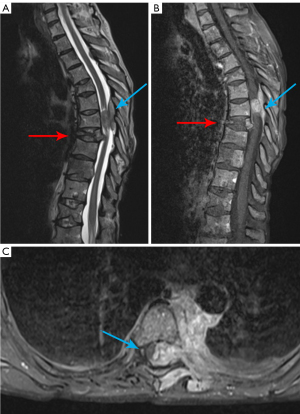
SCC
SCC is reported in about 10% of hematologic patients, with MM being the most common etiology (8%). SCC can be caused by many conditions including vertebral collapse and bone destruction, common in metastasis and MM, infiltrating paravertebral mass, especially in case of lymphoma, leptomeningeal involvement, but also pyogenic spinal infections or epidural hematomas. Usually, the earliest and most common symptom is pain (90%), often with an increasing intensity and progressive disability. Functional symptoms and signs, such as, weakness, sensory loss and autonomic dysfunctions can also be present (12,38,46). A good physical examination allows to detect the anatomic level of SCC; however, imaging is fundamental to assess the cause, extension, and severity of this condition. Contrast enhanced MR is the most accurate diagnostic tool, allowing imaging of the entire spine. It provides information about the level of SCC. Multilevel involvement is seen in about 30% of patients, presence of spinal cord complications such as edema or ischemia (29,38,39).
Cauda equina syndrome is another severe complication determined by compression of descending lumbar and sacral nerve roots, usually presenting with back pain and a classic clinical triad including saddle anesthesia, bowel and/or bladder dysfunction, and lower extremity weakness. It is considered as a diagnostic and surgical emergency because a delay in diagnosis could result in substantial morbidity (29,46).
Vascular
Due to their often-associated state of impaired coagulation, hematological patients have a higher risk of developing acute cerebrovascular conditions, such as posterior reversible encephalopathy syndrome (PRES), intracerebral and subarachnoid hemorrhage, microbleeds, ischemic stroke, and dural sinus and cerebral vein thrombosis (CVT) (Figures 6-8). Hypercoagulability state or bleeding diathesis can be a consequence of altered coagulation factors by interference with production or accelerated breakdown, either due to the disease itself or as a side-effect of chemotherapeutic agents (30,47,48).
PRES
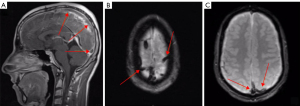
PRES is a rare neurologic disorder often involving parieto-occipital regions, determined by the inadequate autoregulation of posterior circulation in response to sudden increase of systemic blood pressure (Figure 6). Hypertensive peaks can exceed cerebral blood flow autoregulation capacity leading to hyperperfusion, with consequent blood-brain barrier breakdown and vasogenic edema, usually without infarction. The most common symptoms are headache, focal neurological deficits, seizure, and visual disturbances, presenting with a rapid onset (47,49).
Intracerebral hemorrhage (ICH)
ICH is a potentially fatal cerebrovascular complication. It is the second most common neurological complication in adult patients with HM, accounting for 70% of all cerebrovascular diseases in patients with acute leukemia (i.e., acute promyelocytic—APL, monocytic and ALL) and 10–20% of leukemic death. It may result from thrombocytopenia, which is the main risk factor, disseminated intravascular coagulopathy (DIC), common in APL, blast crisis or hyperleukocytosis (2,50). There are multiple underlying pathophysiologic mechanisms that can contribute to develop this condition, including aneurysmal dilatation and bleeding due to extreme leukocytosis leading to hyperviscosity and sludging of blast cells at the venous or capillary bed (e.g., acute leukemia); clotting systems imbalance due to liver infiltration by leukemia cells; thrombocytopenia due to immune mediate platelet destruction or chemotherapy; or impaired platelets function (i.e., myeloproliferative disorders) or aggregation (i.e., elevate paraprotein) (2). Headache and altered mental status are the most common clinical presentations (50).
APL typically causes thrombocytopenia and DIC, with a high risk of ICH as a major cause of death. Moreover, drugs like Ibrutinib used for chronic lymphocytic leukemia (CLL) can also cause thrombocytopenia and several platelet inhibitory mechanisms as a side-effect, possibly contributing to hemorrhage. This can be either intra- or extra-axial (i.e., epi- or sub-dural, subarachnoid, intraventricular or intracerebral) and often with multiple foci. The presence of ICH deserves a close radiological and clinical follow-up (50). In case of ICH in patients with HM, management is based on thrombocytopenia correction with platelets transfusion, coagulopathy with fresh frozen plasma or prothrombin complex, and hypertension control. Surgical treatment is for patients with severe mass effect, presence or imminent risk of brain herniation, intraventricular ICH extension, and hypertensive hydrocephalus (50).
Cerebral infarction
Less common than hemorrhage in HM patients, cerebral infarction can be caused by intravascular coagulation, tumor emboli, septic emboli (e.g., Aspergillus), and arterial compression/infiltration by tumor cells. Acute leukemias can cause ischemic stroke or hemorrhagic stroke and venous infarction. Drugs such as L-asparaginase, used to treat ALL and acute myeloid leukemia are known to produce numerous hemostatic effects leading to reduced synthesis of hemostasis proteins (i.e., proteins S, C and anti-thrombin III) potentially triggering cerebrovascular disorders (Figure 8) (30). The treatment in this setting is quite complex and includes administration of anticoagulants, according to the platelets value, and anti-thrombin III concentrate and cryoprecipitate together with serial CT or MRI follow-up (30,51).
Cerebral venous thrombosis
HM are often prothrombotic conditions, due to thrombocythemia, hypercoagulability, leukostasis, clotting factor deficiencies and chemotherapeutic effect, such as the case of L-asparaginase in patients with ALL. This condition can cause dural venous, cortical vein or deep CVT (Figure 8). Hemorrhage due to venous infarction of the adjacent brain structures may also be present accordingly. Frequently neurological signs and symptoms are present, such as headaches, altered conscious state, papilledema, focal neurological deficits and seizures (48).
This serious condition needs to be promptly treated with anticoagulants and closely monitored.
Immune-related
Immunodeficiency often occurs in patients affected by HM, as a consequence of systemic involvement by the disease itself and/or as side-effects of antineoplastic or immunosuppressive drugs. Immunocompromised patients are on one hand at higher risk of developing CNS infections and on the other hand of disorders like immune reconstitution inflammatory syndrome, possibly leading to worsening of the clinical picture often with acute onset of signs and symptoms (Figures 9,10) (16,17,19).
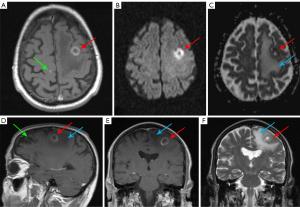
CNS infections
Patients with HM are at increased risk of CNS infections, due to abnormal immune function or treatment-related immunosuppression. CSF analysis and biopsy are the reference exams to obtain the diagnosis. However, neuroimaging plays a fundamental role in guiding the diagnosis and enabling fast patient management.
In patients with a septic state, but also in case of headache, neutropenic fever, personality change, delirium or seizure, CNS infections must be considered (Table 2) (18).
Viral infections can also cause myelitis, and, in such cases, MRI may show increased signal on T2-weighted images and enhancement of the spinal cord. Aspergillus and Toxoplasma gondii are the predominant causative agents of CNS infections in immunocompromised patients, especially in the context of allogeneic hematopoietic stemcell transplantation. The anatomic location and extension of infection are important variables influencing outcome. The treatment is usually based on antifungal amphotericin B (52). Bacterial CNS infections are less commonly diagnosed in patients with HM, but more frequently occur after neurosurgical interventions. In case of suspected intracranial tuberculosis, MRI of the entire neuraxis should be performed to fully investigate brain and spinal involvement (16,17,53).
These conditions should be diagnosed promptly and treated with antimicrobial, antifungal or antiviral therapies.
Immune reconstitution inflammatory syndrome (IRIS)
IRIS is a paradoxical clinical worsening often seen in immunodeficient patients affected by John Cunningham (JC) virus-related progressive multifocal leukoencephalopathy (PML) (19,54).
It is due to restoration of immunity where the immune system abruptly responds to the infection itself, producing an excessive inflammatory state. IRIS has been also described in patients affected by HM previously treated with natalizumab or rituximab (Figure 10) or rarely in immunocompetent patients (20,21,55).
PML primarily manifests at MRI with bilateral and asymmetrical lesions usually located in the subcortical white matter, hyperintense on T2 weighted and FLAIR sequences, without mass effect and contrast enhancement. However, isolated, or associated involvement of the basal ganglia, external capsule, and posterior fossa structures (cerebellum and brainstem) may be seen as well. In IRIS, patchy gadolinium enhancement or mass effect on MRI, atypical findings in PML, in association with worsening neurologic symptoms, are usually present. Moreover, a punctate pattern with milky way appearance has been described in some patients (20,21,55).
Immune reaction to treatment
The introduction in the clinical setting of new treatment leads to the development of new neurological complications. Chimeric antigen receptor (CAR) T cellular therapy is a treatment used in case of B-cell HM, which, however, can lead to neurotoxicity. The pathophysiology is related to endothelial activation, with subsequent DIC associated to blood-brain barrier (BBB) dysfunction and passive diffusion of cytokines into the brain (31,54). This condition can be symptomatic with confusion, delirium, cognitive impairment, and language disturbance such as expressive aphasia.
Brain MRI is usually normal, but thalami and brainstem hyperintensity on T2/fluid-attenuated inversion recovery (FLAIR) sequences, along with cerebral edema have been reported. Steroids and immunosuppressive drugs are treatment choice (31).
Other medications, such as blinatumomab, cytarabine or ifosfamide can lead to neurotoxicity, caused by different mechanisms; sometimes these conditions can present with diffuse cerebral white-matter abnormalities on MRI.
Radiation-induced changes
Radiation-induced changes can cause cognitive impairment and can be categorized in:
- Acute or early delayed injury and long-term sequelae, that present on T2-weighted/FLAIR MRI sequences as confluent, periventricular white matter hyperintensities.
- Late delayed radiation necrosis, that can mimic recurrent disease, appearing as a mass with variable enhancement and central necrosis; hence correlation with timing and treatment details is fundamental to ensure an accurate interpretation of findings. Moreover, advanced MRI techniques such as diffusion tensor imaging (DTI), dynamic susceptibility-weighted contrast (DSC), dynamic contrast-enhanced (DCE), and arterial spin labeling (ASL) brain perfusion imaging can be helpful in discriminating between recurrent neoplasms and radiation necrosis in patients with history of brain irradiation (32,56).
Conclusions
Adult patients with HM are at risk of developing acute conditions involving the nervous system. Clinically, there can be generalized and nonspecific symptoms, which may cause rapid deterioration and present as an emergency. Since CNS involvement in patients affected by HM often has a poor prognosis, a thorough knowledge of the etiology as well as the expected CT and MRI findings is key in making a prompt and correct diagnosis in order to start the most appropriate treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: With the arrangement by the Guest Editors and the editorial office, this article has been reviewed by external peers.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1201/coif). The special issue “Imaging of Aging and Age-Related Disorders” was commissioned by the editorial office without any funding or sponsorship. CAM served as the unpaid Guest Editor of the issue and serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Polyatskin IL, Artemyeva AS, Krivolapov YA. Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition):lymphoid tumors. Arkh Patol 2019;81:59-65.
- Mauermann ML. Neurologic Complications of Lymphoma, Leukemia, and Paraproteinemias. Continuum (Minneap Minn) 2017;23:669-90. [Crossref] [PubMed]
- Wang J, Yan H, Tian S, Qin L, Ma Y. Unexpected discovery of prostatic diffuse large B-cell lymphoma after thulium laser vaporization in a patient with Waldenstrom macroglobulinemia. Quant Imaging Med Surg 2022;12:862-7. [Crossref] [PubMed]
- Ferro JM, Infante J. Cerebrovascular manifestations in hematological diseases: an update. J Neurol 2021;268:3480-92. [Crossref] [PubMed]
- Lasaponara S, Mauro F, Carducci F, Paoletti P, Tombini M, Quattrocchi CC, Mallio CA, Errante Y, Scarciolla L, Ben-Soussan TD. Increased Alpha Band Functional Connectivity Following the Quadrato Motor Training: A Longitudinal Study. Front Hum Neurosci 2017;11:282. [Crossref] [PubMed]
- Keraliya AR, Krajewski KM, Giardino AA, Tirumani SH, Shinagare AB, Ramaiya NH, Jagannathan JP. Imaging of Nervous System Involvement in Hematologic Malignancies: What Radiologists Need to Know. AJR Am J Roentgenol 2015;205:604-17. [Crossref] [PubMed]
- Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 2011;32:984-92. [Crossref] [PubMed]
- Radbruch A, Wiestler B, Kramp L, Lutz K, Bäumer P, Weiler M, Roethke M, Sahm F, Schlemmer HP, Wick W, Heiland S, Bendszus M. Differentiation of glioblastoma and primary CNS lymphomas using susceptibility weighted imaging. Eur J Radiol 2013;82:552-6. [Crossref] [PubMed]
- Roth P, Korfel A, Martus P, Weller M. Pathogenesis and management of primary CNS lymphoma. Expert Rev Anticancer Ther 2012;12:623-33. [Crossref] [PubMed]
- Gerstner ER, Batchelor TT. Primary central nervous system lymphoma. Arch Neurol 2010;67:291-7. [Crossref] [PubMed]
- Giannini C, Dogan A, Salomão DR. CNS lymphoma: a practical diagnostic approach. J Neuropathol Exp Neurol 2014;73:478-94. [Crossref] [PubMed]
- Albagoush SA, Azevedo AM. Multiple Myeloma. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2022.
- Chen CI, Masih-Khan E, Jiang H, Rabea A, Cserti-Gazdewich C, Jimenez-Zepeda VH, Chu CM, Kukreti V, Trudel S, Tiedemann R, Tsang R, Reece DE. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol 2013;162:483-8. [Crossref] [PubMed]
- Amos B, Agarwal A, Kanekar S. Imaging of Multiple Myeloma. Hematol Oncol Clin North Am 2016;30:843-65. [Crossref] [PubMed]
- Paludo J, Painuly U, Kumar S, Gonsalves WI, Rajkumar V, Buadi F, Lacy MQ, Dispenzieri A, Kyle RA, Mauermann ML, McCurdy A, Dingli D, Go RS, Hayman SR, Leung N, Lust JA, Lin Y, Gertz MA, Kapoor P. Myelomatous Involvement of the Central Nervous System. Clin Lymphoma Myeloma Leuk 2016;16:644-54. [Crossref] [PubMed]
- Carmo RLD, Alves Simão AK, Amaral LLFD, Inada BSY, Silveira CF, Campos CMS, Freitas LF, Bonadio V, Marussi VHR. Neuroimaging of Emergent and Reemergent Infections. Radiographics 2019;39:1649-71. [Crossref] [PubMed]
- Schmidt-Hieber M, Silling G, Schalk E, Heinz W, Panse J, Penack O, Christopeit M, Buchheidt D, Meyding-Lamadé U, Hähnel S, Wolf HH, Ruhnke M, Schwartz S, Maschmeyer G. CNS infections in patients with hematological disorders (including allogeneic stem-cell transplantation)-Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann Oncol 2016;27:1207-25. [Crossref] [PubMed]
- Ogata M, Fukuda T, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant 2015;50:1030-6. [Crossref] [PubMed]
- Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009;72:1458-64. [Crossref] [PubMed]
- Krey L, Raab P, Sherzay R, Berding G, Stoll M, Stangel M, Wegner F. Severe Progressive Multifocal Leukoencephalopathy (PML) and Spontaneous Immune Reconstitution Inflammatory Syndrome (IRIS) in an Immunocompetent Patient. Front Immunol 2019;10:1188. [Crossref] [PubMed]
- Al-Tawfiq JA, Banda RW, Daabil RA, Dawamneh MF. Progressive multifocal leukoencephalopathy (PML) in a patient with lymphoma treated with rituximab: A case report and literature review. J Infect Public Health 2015;8:493-7. [Crossref] [PubMed]
- Wang Y, Pan ZC, Zhu L, Ma YY, Zhang MC, Wang L, Zhao WL, Yan FH, Song Q. The characteristic computed tomography findings of pulmonary B-cell non-Hodgkin's lymphoma and their role in predicting patient survival. Quant Imaging Med Surg 2021;11:772-83. [Crossref] [PubMed]
- Peters S, Knöß N, Wodarg F, Cnyrim C, Jansen O. Glioblastomas vs. lymphomas: more diagnostic certainty by using susceptibility-weighted imaging (SWI). Rofo 2012;184:713-8. [Crossref] [PubMed]
- Kawase Y, Yamamoto Y, Kameyama R, Kawai N, Kudomi N, Nishiyama Y. Comparison of 11C-methionine PET and 18F-FDG PET in patients with primary central nervous system lymphoma. Mol Imaging Biol 2011;13:1284-9. [Crossref] [PubMed]
- Puranik AD, Boon M, Purandare N, Rangarajan V, Gupta T, Moiyadi A, Shetty P, Sridhar E, Agrawal A, Dev I, Shah S. Utility of FET-PET in detecting high-grade gliomas presenting with equivocal MR imaging features. World J Nucl Med 2019;18:266-72. [Crossref] [PubMed]
- Schmitz N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH, Glass B, Scott DW, Gascoyne RD, Connors JM, Ziepert M, Pfreundschuh M, Loeffler M, Savage KJ. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J Clin Oncol 2016;34:3150-6. [Crossref] [PubMed]
- Sun M, Cheng J, Ren C, Zhang Y, Li Y, Li Y, Zhang S. Quantitative whole-body MR imaging for assessment of tumor burden in patients with multiple myeloma: correlation with prognostic biomarkers. Quant Imaging Med Surg 2021;11:3767-80. [Crossref] [PubMed]
- Ji X, Huang W, Dong H, Shen Z, Zheng M, Zou D, Shen W, Xia S. Evaluation of bone marrow infiltration in multiple myeloma using whole-body diffusion-weighted imaging and T1-weighted water-fat separation Dixon. Quant Imaging Med Surg 2021;11:641-51. [Crossref] [PubMed]
- Shi J, Jia L, Yuan W, Shi G, Ma B, Wang B, Wu J. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop 2010;81:391-5. [Crossref] [PubMed]
- Eden D, Hipkins R, Bradbury CA. Cerebral Thrombotic Complications Related to l-Asparaginase Treatment for Acute Lymphoblastic Leukemia: Retrospective Review of 10 Cases. Clin Appl Thromb Hemost 2016;22:589-93. [Crossref] [PubMed]
- Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J, Chung D, Harju-Baker S, Özpolat T, Fink KR, Riddell SR, Maloney DG, Turtle CJ. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017;7:1404-19. [Crossref] [PubMed]
- Masch WR, Wang PI, Chenevert TL, Junck L, Tsien C, Heth JA, Sundgren PC. Comparison of Diffusion Tensor Imaging and Magnetic Resonance Perfusion Imaging in Differentiating Recurrent Brain Neoplasm From Radiation Necrosis. Acad Radiol 2016;23:569-76. [Crossref] [PubMed]
- Quattrocchi CC, Errante Y, Mallio CA, Santini D, Tonini G, Zobel BB. Brain metastatic volume and white matter lesions in advanced cancer patients. J Neurooncol 2013;113:451-8. [Crossref] [PubMed]
- Quattrocchi CC, Giona A, Di Martino A, Gaudino F, Mallio CA, Errante Y, Occhicone F, Vitali MA, Zobel BB, Denaro V. Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: a retrospective MRI study. Musculoskelet Surg 2015;99:159-63. [Crossref] [PubMed]
- Perkins AS, Friedberg JW. Burkitt lymphoma in adults. Hematology Am Soc Hematol Educ Program 2008;341-8. [Crossref] [PubMed]
- Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 2013;88:360-76. [Crossref] [PubMed]
- Ponzoni M, Ferreri AJ, Campo E, Facchetti F, Mazzucchelli L, Yoshino T, Murase T, Pileri SA, Doglioni C, Zucca E, Cavalli F, Nakamura S. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol 2007;25:3168-73. [Crossref] [PubMed]
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003;15:211-7. [Crossref] [PubMed]
- Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J 2020;10:94. [Crossref] [PubMed]
- Wang X, Niu F, Ren J, He P. (18)F-FDG PET/CT for the detection of extensive bone relapse in acute lymphoblastic leukemia with TCF3-PBX1 fusion after hematopoietic stem cell transplantation. Quant Imaging Med Surg 2022;12:4002-4. [Crossref] [PubMed]
- Chamberlain MC. Neoplastic myelopathies. Continuum (Minneap Minn) 2015;21:132-45. [Crossref] [PubMed]
- Greco F, Mallio CA. Relationship between visceral adipose tissue and genetic mutations (VHL and KDM5C) in clear cell renal cell carcinoma. Radiol Med 2021;126:645-51. [Crossref] [PubMed]
- Pinto VL, Tadi P, Adeyinka A. Increased Intracranial Pressure. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2021.
- Holbrook J, Saindane AM. Imaging of Intracranial Pressure Disorders. Neurosurgery 2017;80:341-54. [Crossref] [PubMed]
- Pinto VL, Tadi P, Adeyinka A. Increased Intracranial Pressure. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2022.
- Long B, Koyfman A, Gottlieb M. Evaluation and management of cauda equina syndrome in the emergency department. Am J Emerg Med 2020;38:143-8. [Crossref] [PubMed]
- Gewirtz AN, Gao V, Parauda SC, Robbins MS. Posterior Reversible Encephalopathy Syndrome. Curr Pain Headache Rep 2021;25:19. [Crossref] [PubMed]
- Canedo-Antelo M, Baleato-González S, Mosqueira AJ, Casas-Martínez J, Oleaga L, Vilanova JC, Luna-Alcalá A, García-Figueiras R. Radiologic Clues to Cerebral Venous Thrombosis. Radiographics 2019;39:1611-28. [Crossref] [PubMed]
- Parasher A, Jhamb R. Posterior reversible encephalopathy syndrome (PRES): presentation, diagnosis and treatment. Postgrad Med J 2020;96:623-8. [Crossref] [PubMed]
- Raghavan A, Wright CH, Wright JM, Jensen K, Malloy P, Elder T, Burant C, Sajatovic M, Hoffer A. Outcomes and Clinical Characteristics of Intracranial Hemorrhage in Patients with Hematologic Malignancies: A Systematic Literature Review. World Neurosurg 2020;144:e15-24. [Crossref] [PubMed]
- Bekelis K, Fisher ES, Labropoulos N, Zhou W, Skinner J. Variations in the intensive use of head CT for elderly patients with hemorrhagic stroke. Radiology 2015;275:188-95. [Crossref] [PubMed]
- Gletsou E, Ioannou M, Liakopoulos V, Tsiambas E, Ragos V, Stefanidis I. Aspergillosis in immunocompromised patients with haematological malignancies. J BUON 2018;23:7-10.
- Lim EA, Gnanadurai R, Ruffle JK, Lee H, Miller RF, Hyare H. Neuroimaging of CNS infection in haematological malignancy: important signs and common diagnostic pitfalls. Clin Radiol 2021;76:470.e1-470.e12. [Crossref] [PubMed]
- Bowen L, Nath A, Smith B. CNS immune reconstitution inflammatory syndrome. Handb Clin Neurol 2018;152:167-76. [Crossref] [PubMed]
- Trunfio M, Manini C, Trentalange A, Boghi A, Audagnotto S, Imperiale D, Taraglio S, Bonora S, Di Perri G, Calcagno A. The "milky way" galaxy of HIV-related central nervous system immune reaction syndromes. J Neurovirol 2019;25:887-92. [Crossref] [PubMed]
- Ye J, Bhagat SK, Li H, Luo X, Wang B, Liu L, Yang G. Differentiation between recurrent gliomas and radiation necrosis using arterial spin labeling perfusion imaging. Exp Ther Med 2016;11:2432-6. [Crossref] [PubMed]


