Isolated macrocerebellum: description of six cases and literature review
Introduction
Macrocerebellum is an entity introduced by Bodensteiner et al. in 1997 (1), described as an isolated and abnormal increase of the cerebellum (CB) size without morphological or signal abnormalities (2). An abnormal enlargement of the CB can be also seen in some well-known metabolic and genetic diseases or clinical syndromes such as Lhermitte-Duclos (3,4), Sotos syndrome (5), Costello syndrome (6), Williams syndrome (7), Alexander disease (8), mucopolysaccharidoses (9) and fucosidosis (10).
The aim of this paper is to describe the neuroradiological and clinical features of six patients with macrocerebellum stressing the differences between these cases of isolated macrocerebellum and the syndromic/metabolic conditions associated with cerebellar enlargement.
In our case series the clinical suspicion of macrocerebellum was confirmed using volumetric analysis and comparison with normal values (age-matched) available in literature (11,12). An extensive literature review was also performed.
Methods
Patient cohort
From December 2011 to March 2014, among 950 paediatric patients that underwent a magnetic resonance scan of the brain in our department, in six subjects an abnormal increase of the cerebellar volume was suspected. Four of these patients (all males) were referred to the diagnostic imaging department from paediatrics while the remaining two (one male and one female) were from the Child and Adolescent Psychiatry Department. The reason for the scan was developmental delay (n=4), developmental delay and oculomotor apraxia (n=1) and Rubinstein-Taybi syndrome (RTS) (n=1), which is a genetic syndrome associated with microdeletion of the chromosome 16 (in this case CREBBP gene was mutated) and characterized by short stature, abnormal facies, hypotonia, moderate mental delay and marked speech delay (13). Two of the four patients with developmental delay only, had a history of urgent caesarean section for pre-eclampsia (n=1) and foetal bradycardia in twin pregnancy (n=1). In both these patients there were no major abnormalities demonstrated at postnatal examination. The ages of the patients at the time of the scan were 1 to 8 years (mean 4.4 years). The syndromic and metabolic diseases characterized by known association with cerebellar enlargement (see discussion) were excluded in all the patients.
Imaging studies
All the imaging studies were performed with Philips Gyroscan Intera 1.5 Tesla MR scanner (Philips Medical System, Best, Netherlands) with axial T2 weighted imaging and/or axial T2 FLAIR (slice thickness 4 mm), coronal T2 weighted imaging (slice thickness 3 mm), sagittal T2 weighted imaging (slice thickness 4 mm), axial diffusion weighted imaging (DWI) and sagittal 3D T1 weighted imaging (iso-tropic voxel: 0.5 mm) with reformats in coronal and axial planes (thickness: 1 mm/gap: 0 mm).
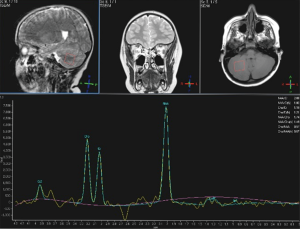
In only one patient (male, 6-year-old, developmental delay and history of urgent caesarean section for pre-eclampsia), a MR spectroscopy of the deep cerebellar white matter (WM) (single voxel, TE 288 ms) was performed.
Two expert neuroradiologists (Alessandra D’Amico and Ferdinando Caranci, with 20 and 25 years of experience respectively) confirmed, in consensus, that the CB of all the six patients was abnormally enlarged. The other diseases with abnormal enlarged CB discussed above were excluded clinically.
Volumetric and statistical analysis
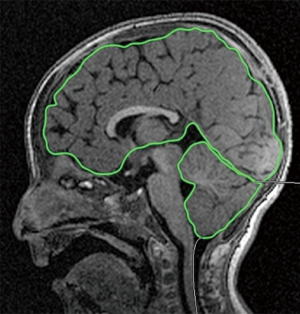
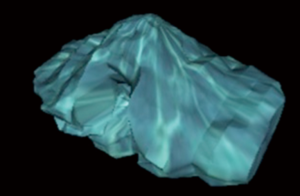
The 3D T1 weighted sequences, available in all the patients, were used to calculate cerebellar and cerebral volumes. Two radiology residents (5th and 3rd years of residency) manually drew the cerebellar and cerebral outlines as described by Bodensteiner et al. (1) and by Pichiecchio et al. (14). ROI placement and volume analysis were performed using OsiriX MD Dicom Viewer (15) which has a specific algorithm for volumetric calculation (OsiriX Compute Volume Plugin) in which slice thickness and number of slices are considered (Figures 1,2). Since the sequence used for volumetric calculation was a 3D volumetric sequence with no gap between the slices, there was no need to add an intergap correction to the calculation.
The ratios between (I) volume of the CB and volume of the supratentorial structures (STB) and (II) volume of the CB and the sum of CB and STB (WB) were calculated in order to normalize the absolute values obtained and compared with the normal values present in literature (1). A two tailed student’s t-test for independent samples with a 99% confidence interval and 39 degrees of freedom was used to compare these ratios.
Results
Ratios CB/STB (cerebellar volume/supra-tentorial brain volume) and CB/WB [cerebellar volume/whole brain volume (i.e., cerebellar volume + supra-tentorial brain volume] obtained are shown in the Table 1. There is an increased cerebellar volume relatively to the STB volume (“t”: 6.9518; P<0.001) and to the WB (“t”: 7.1415; P<0.001) volume in comparison to the normal controls available in literature (1). Thus, the quantitative results confirmed the qualitative diagnosis of macrocerebellum (Figure 3).
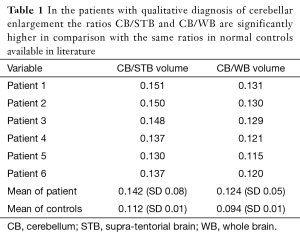
Full table
A possible bias related to the normal values used as reference is that these were obtained from controls aged between 9 and 24 months (1). The patient No. 4, 5 and 6 were respectively 6-, 8- and 8-year-old, so the ratios obtained in these patients were not correlated to normal controls of the same age range. In order to overcome this possible bias, we double-checked the ratios obtained in these three patients with ratios obtained from normal absolute volumetric values in normal controls aged between 4.5 and from 18 years obtained from literature (12). Calculating CB/STB and CB/WB using these data we obtained the following ratios: for males CB/STB =0.119 and CB/WB =0.107; for females CB/STB =0.121 and CB/WB =0.108. Also using these new values as normal references, the cerebellar volume of the patients 4 and 5 (males) and 6 (female) were significantly increased.
In one patient (patient No. 3, developmental delay and oculomotor apraxia), a follow-up MR was performed after 2 years (age of the patient 3 years and 3 months) showing grossly stable ratios (CB/STB: 0.148; CB/WB: 0.129).
In patient No. 4 (male, 6-year-old, developmental delay and history of urgent caesarean section for pre-eclampsia), a MR spectroscopy of the deep cerebellar WM (single voxel, TE 288 ms) was performed (Figure 4) with no abnormalities demonstrated.
Apart from the macrocerebellum, other abnormal neuroradiological findings were identified in some of the patients.
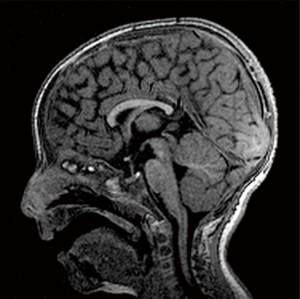
The patient 1, with genetic diagnosis of RTS, also had a delay of the myelination, some peri-ventricular areas of abnormal WM signal and hypoplasia of the corpus callosum (Figure 5). In the same patient a CT of petrous bones performed after the MR scan, demonstrated a cochlear malformation incomplete partition type 2 (IP type 2). This was consistent with right-sided neurosensorial deafness.
Some non-specific areas of abnormal peri-trigonal WM signal were demonstrated in patients No. 4 and 5. Patient No. 6 had a mild dilatation of the supratentorial ventricular system and prominent flow artefact thorough the cerebral aqueduct but no signs of acute hydrocephalus.
Discussion
The evidence of cerebellar enlargement is a rare finding on imaging and is often associated with the presence of a definite syndromic condition (such as Lhermitte-Duclos syndrome, Sotos syndrome, Costello syndrome, Alexander disease, Williams syndrome and fucosidosis) (2). On the other hand, cerebellar hypoplasia/atrophy is a more frequent finding in paediatric patients with genetic, metabolic, toxic or infective diseases that involve the central nervous system (2,16).
The main difference among macrocerebellum and Lhermitte-Duclos Disease (dysplastic gangliocytoma of the CB) (3) is that the latter shows involvement of one cerebellar hemisphere only and of the vermis, with structural abnormalities in the folia, resulting in a tumour-like lesion which expands and replace the normal cerebellar architecture, with typical “tigroid” appearance on MRI study (17). Sotos syndrome shows ventriculomegaly, enlarged pericerebral spaces, abnormalities of the midline (such as dysmorphic corpus callosum), increased volume of the posterior fossa and gray matter (GM) heterotopias (18). The physical and genetic features of these patients are typical for Sotos syndrome and not present in any patient with macrocerebellum. Costello syndrome is associated with several brain abnormalities, such as relative macrocrania, ventriculomegaly and abnormalities of the posterior fossa with a progressive and diffuse cerebellar enlargement; specific genetic and phenotypical features interesting several organs and tissues are necessary to make diagnosis. Alexander disease is characterized by a dramatic and diffuse loss of myelin in both the cerebral hemispheres, in the CB and brainstem with associated increase of the density of the astrocytes from which derives an increase in the brain size (megalencephaly) (19). In 2005, Van Der Knaap and colleagues described a variant in which the posterior fossa was mainly involved, with tumour-like lesions and increase of the volume of the CB and of the brainstem (8). Overtime the neuronal structures involved became atrophic. In these cases, the abnormal signal in the cerebellar WM and the dramatic clinical evolution allow the differential diagnosis with macrocerebellum. Neuroradiological studies in patients with Williams syndrome show reduction of both cerebellar and cerebral volume with increase cerebellar/cerebral volumes ratio (20). Thus, the apparent increase of the cerebellar size is relative to a reduction of the cerebrum. Fucosidosis shows diffuse and marked symmetrical signal abnormalities at the level of the basal ganglia, subcortical WM and cerebellar WM (10). However, five cases of fucosidosis have been described in which there was an increased cerebellar volume with progressive cerebellar atrophy in follow-up examinations (21).
Isolated macrocerebellum, not related to any one of the syndromic conditions described above, is a much more rare entity, reported in only two cohorts of patients (1,2) and two case reports (14,22). However, being necessarily a volumetric confirmation of the subjective suspect of macrocerebellum on images, it is possible that the entity has been historically underestimated. Bodensteiner et al. first described this neuroradiological entity in 1997 (1); they reported an abnormal increase of the cerebellar volume, confirmed by the quantitative analysis and comparison with normal controls in four subjects. Their patients were aged 9 to 24 months (three males and one female) and all had mental and motor delay, ocular apraxia, facial dysmorphism and delay in myelination. The cerebellar enlargement seemed to be mostly related to increase of the size of the cerebellar hemisphere over the vermis. Other than the volumetric enlargement, the CB of these patients didn’t show any signal or morphological abnormality. The authors hypothesized that, in presence of a low response from the cerebrum to normal neuro-trophic stimulation (for unknown reasons), a normal CB could react with a volumetric increase to the oversecretion of growing factors. Thus the cerebellar enlargement would represent an epiphenomenon and not a primary manifestation.
In 2011, Pichiecchio et al. (14) described a case of a patient with agenesis of the corpus callosum, microcephaly, strabismus, muscular hypertonia, facial dysmorphisms and mental delay. This patient did not show any sign of macrocerebellum at the first magnetic resonance performed at birth that showed instead a callosal agenesis, dilated lateral ventricles and dysmorphic hippocampi. However in a subsequent follow up performed when the child was 19-month-old, there was an increase of the cerebellar size confirmed at the volumetric analysis. The fact that the macrocerebellum was not present at birth but developed overtime is in keeping with the hypothesis of macrocerebellum as epiphenomenon. The method used for the measurements and the controls were the same used by Bodensteiner and colleagues.
Poretti et al. (2) described five patients with qualitative diagnosis of macrocerebellum (age range, 7–28 months) of which three (where 3D T1 sequence was available) had a volumetric analysis confirming the volumetric enlargement in comparison with age and gender matched controls. All the subjects in this study had abnormal muscular tone and mental delay, four had dysmorphic features and three also had abnormal ocular movements and seizures. Thus the clinical features of this cohort of patient were similar to those described previously (1,14). Furthermore, four of these patients showed abnormal signal of the cerebral WM, reduction of WM bulk and delay of myelination while no one had abnormal increase of the vermis in comparison to the controls. In all the patients a various degree of ventricular dilatation/hydrocephalus was demonstrated. In contrast to previous studies, together with cerebellar enlargement, there was described marked thickening of the cerebellar grey matter.
Finally, Seeley et al. (22) described a case of macrocerebellum, epilepsy, gut malrotation and mental delay in a child with a specific deletion (16q24.1-q24.2). No other neuroradiological abnormalities were reported.
Imaging findings and clinical features of our cohort are similar to those described previously, particularly all patients had mental and motor delay and three showed abnormal WM signal in the cerebrum. Finally, the patient with clinical diagnosis of Rubinstein-Taybi had hypoplasia of the corpus callosum (such as the patient described by Pichiecchio and colleagues). This patient also had myelination delay and an IP type 2 malformation of the right inner ear.
RTS is a well-defined condition characterized by multiple congenital abnormalities, microcephaly, facial dysmorphism and mental delay (13). Neuroradiological features described so far include reduced WM bulk and callosal dysgenesis, low position of the cerebellar tonsils (Chiari 1) and syringomyelia (23-25). However the presence of Chiari 1 and syrinx were related to abnormalities of the posterior fossa and of the cranio-vertebral junction, not with a volumetric enlargement of the CB. In our case the cranio-vertebral junction and the posterior fossa were normal. Our patient also had hypoplastic corpus callosum, in keeping with the literature, and an IP type 2 malformation of the right ear that, to the best of our knowledge, has never been described before in association with RTS.
Patients No. 4, 5 and 6 underwent a brain MRI when they were 6-, 8- and 8-year-old (older than the other patients described so far). For all of them the clinical indication to the MRI was a non-specific developmental delay. In these patients the signal of the WM was normal, this could be due to the older age of the patients. Indeed, in the only patient described by Bodensteiner et al. (1) with follow-up study, there was a progressive myelin maturation at the most recent MRI (3-year-old), with no associated clinical improvement.
Also in one of the subjects described by Poretti et al. (2), the cerebral WM was reported as normal. So the presence of abnormal cerebral WM seems to be often, but not always, associated with macrocerebellum, while the presence of a certain degree of mental and motor delay is always present.
Regarding the abnormal thickening of the cerebellar GM described by Poretti et al. (2), in our cohort of patients the cerebellar GM was judged to be normal and the significance of this finding remains unclear. We believe that volumetric analysis with segmentation of the different components of the CB (grey matter, WM and liquor) could be useful to clarify the contribution of the cortical thickening to the macrocerebellum.
Conclusions
Macrocerebellum is a neuroradiological entity that can be identified qualitatively and confirmed quantitatively through volumetric analysis. This finding is associated with an abnormal mental and motor development and often with abnormalities of the cerebral WM (including dysgenesis of the corpus callosum) and facial dysmorphisms. It is likely that, with an increased use of volumetric measurements in patients where an enlarged CB is suspected, a larger number of patients with macrocerebellum will be identified. Due to the variability of clinical, radiological and genetic findings, our findings support Bordensteiner’s hypothesis that macrocerebellum could be an epiphenomenon relative to an abnormal cerebral development rather than a distinct nosological entity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bodensteiner JB, Schaefer GB, Keller GM, Thompson JN, Bowen MK. Macrocerebellum: neuroimaging and clinical features of a newly recognized condition. J Child Neurol 1997;12:365-8. [Crossref] [PubMed]
- Poretti A, Mall V, Smitka M, Grunt S, Risen S, Toelle SP, Benson JE, Yoshida S, Jung NH, Tinschert S, Neuhann TM, Rauch A, Steinlin M, Meoded A, Huisman TA, Boltshauser E. Macrocerebellum: significance and pathogenic considerations. Cerebellum 2012;11:1026-36. [Crossref] [PubMed]
- Milbouw G, Born JD, Martin D, Collignon J, Hans P, Reznik M, Bonnal J. Clinical and radiological aspects of dysplastic gangliocytoma (Lhermitte-Duclos disease): a report of two cases with review of the literature. Neurosurgery 1988;22:124-8. [PubMed]
- Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): a malformation, hamartoma or neoplasm? Acta Neurol Scand 2002;105:137-45. [Crossref] [PubMed]
- Schaefer GB, Bodensteiner JB, Buehler BA, Lin A, Cole TR. The neuroimaging findings in Sotos syndrome. Am J Med Genet 1997;68:462-5. [Crossref] [PubMed]
- Gripp KW, Hopkins E, Doyle D, Dobyns WB. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A 2010;152A:1161-8. [Crossref] [PubMed]
- Jones W, Hesselink J, Courchesne E, Duncan T, Matsuda K, Bellugi U. Cerebellar abnormalities in infants and toddlers with Williams syndrome. Dev Med Child Neurol 2002;44:688-94. [Crossref] [PubMed]
- Van Der Knaap MS, Salomons GS, Li R, Franzoni E, Gutiérrez-Solana LG, Smit LM, Robinson R, Ferrie CD, Cree B, Reddy A, Thomas N, Banwell B, Barkhof F, Jakobs C, Johnson A, Messing A, Brenner M. Unusual variants of Alexander's disease. Ann Neurol 2005;57:327-38. [Crossref] [PubMed]
- Alqahtani E, Huisman TA, Boltshauser E, Scheer I, Güngör T, Tekes A, Maegawa GH, Poretti A. Mucopolysaccharidoses type I and II: new neuroimaging findings in the cerebellum. Eur J Paediatr Neurol 2014;18:211-7. [Crossref] [PubMed]
- Galluzzi P, Rufa A, Balestri P, Cerase A, Federico A. MR brain imaging of fucosidosis type I. AJNR Am J Neuroradiol 2001;22:777-80. [PubMed]
- Schaefer GB, Thompson JN Jr, Bodensteiner JB, Gingold M, Wilson M, Wilson D. Age-related changes in the relative growth of the posterior fossa. J Child Neurol 1991;6:15-9. [Crossref] [PubMed]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex 2012;22:1-12. [Crossref] [PubMed]
- Hennekam RC. Rubinstein-Taybi syndrome. Eur J Hum Genet 2006;14:981-5. [Crossref] [PubMed]
- Pichiecchio A, Di Perri C, Arnoldi S, Berardinelli A, Branca V, Balottin U, Bastianello S. Cerebellum enlargement and corpus callosum agenesis: a longitudinal case report. J Child Neurol 2011;26:756-60. [Crossref] [PubMed]
- Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004;17:205-16. [Crossref] [PubMed]
- Poretti A, Boltshauser E. Cerebellar hypoplasia. In: Boltshauser E, Schmahmann JD, edtors. Cerebellar disorders in children. London: Mac Keith Press, 2012:121-34.
- Cianfoni A, Wintermark M, Piludu F, D'Alessandris QG, Lauriola L, Visocchi M, Colosimo C. Morphological and functional MR imaging of Lhermitte-Duclos disease with pathology correlate. J Neuroradiol 2008;35:297-300. [Crossref] [PubMed]
- Horikoshi H, Kato Z, Masuno M, Asano T, Nagase T, Yamagishi Y, Kozawa R, Arai T, Aoki M, Teramoto T, Omoya K, Matsumoto N, Kurotaki N, Shimokawa O, Kurosawa K, Kondo N. Neuroradiologic findings in Sotos syndrome. J Child Neurol 2006;21:614-8. [Crossref] [PubMed]
- Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. Alexander disease. J Neurosci 2012;32:5017-23. [Crossref] [PubMed]
- Chiang MC, Reiss AL, Lee AD, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, Toga AW, Thompson PM. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage 2007;36:1096-109. [Crossref] [PubMed]
- Kau T, Karlo C, Güngör T, Prietsch V, Kellenberger CJ, Scheer I, Boltshauser E. Increased cerebellar volume in the early stage of fucosidosis: a case control study. Neuroradiology 2011;53:509-16. [Crossref] [PubMed]
- Seeley AH, Durham MA, Micale MA, Wesolowski J, Foerster BR, Martin DM. Macrocerebellum, epilepsy, intellectual disability, and gut malrotation in a child with a 16q24.1-q24.2 contiguous gene deletion. Am J Med Genet A 2014;164A:2062-8. [Crossref] [PubMed]
- Sener RN. Rubinstein-Taybi syndrome: cranial MR imaging findings. Comput Med Imaging Graph 1995;19:417-8. [Crossref] [PubMed]
- Parsley L, Bellus G, Handler M, Tsai AC. Identical twin sisters with Rubinstein-Taybi syndrome associated with Chiari malformations and syrinx. Am J Med Genet A 2011;155A:2766-70. [Crossref] [PubMed]
- Wójcik C, Volz K, Ranola M, Kitch K, Karim T, O'Neil J, Smith J, Torres-Martinez W. Rubinstein-Taybi syndrome associated with Chiari type I malformation caused by a large 16p13.3 microdeletion: a contiguous gene syndrome? Am J Med Genet A 2010;152A:479-83. [Crossref] [PubMed]