Disease-specific cardiovascular positron emission tomography/magnetic resonance imaging: a brief review of the current literature
Introduction
More than two decades ago, simultaneous PET/CT was conceptualized, and the first commercial PET/CT scanner became available in 2001. The addition of CT to PET allowed the PET images to be interpreted with anatomic correlation, improving diagnostic confidence and accuracy. Such was the success of PET/CT that by 2006, PET-only scanners were no longer obtainable. In some ways, the advance of positron emission tomography/magnetic resonance (PET/MR) seems to follow a similar path. But does it?
In its early days, PET/CT clinical workload was mostly driven by oncologic applications. Nevertheless, owing to its high resolution and dynamic functional imaging, PET/CT gradually found its way in various cardiovascular applications, including stress myocardial perfusion imaging, myocardial viability imaging, and coronary artery imaging. PET/CT is now considered gold standard for myocardial perfusion imaging (1). Clinical utility in less-prevalent cardiovascular conditions such as sarcoidosis, amyloidosis, etc., are less well-defined. On the other hand, cardiac MR provides dynamic functional assessment of the heart, valvular assessment, and great vessel assessment. With the advance of gadolinium and late gadolinium enhancement (LGE) imaging, it also finds its way in assessing myocardial perfusion and infarcts. It is also the imaging modality of choice for complex congenital heart diseases.
Unlike standalone cardiac MR and cardiac PET/CT, which have found their respective niches in clinical cardiology, until now cardiac PET/MR remains mostly an experimental and preclinical investigative tool (2). In theory, PET/MR combines the quantitative measurement of PET with dynamic functional and anatomic assessment of MR to offer a comprehensive “one-stop” cardiovascular examination. In practice, questions remain what incremental values a cardiac PET/MR examination offers above current standard-of-care imaging modalities. There is also concern of cost-effectiveness of PET/MR, i.e., whether PET/MR offers incremental benefit over either PET/CT or MR alone.
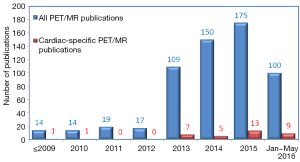
Hybrid PET/MR imaging was approved by the U.S. Food and Drug Administration for purchase in 2011 (1). Since then, we saw a rapidly increasing body of literature investigating its clinical use (Figure 1). Cardiovascular-specific PET/MR publications was lacking initially, but had steadily picked up its pace in the last several years, even though it still represents roughly only 6% of total PET/MR publications. In this brief review, we look at several areas of potential cardiovascular PET/MR applications, including in the assessment of coronary artery disease (CAD), carotid atherosclerosis, infiltrative, inflammatory or hereditary heart diseases, myocarditis, vasculitis, and cardiac mass assessment.
Coronary artery disease
Detection of CAD: PET/MR stress test
CAD affects roughly 23.5% of all U.S. population according to the Center for Disease Control, and remains the leading cause of death in the United States (3). Given its high prevalence, CAD is an obvious area of research to investigate the potential use of simultaneous PET/MR imaging. Early detection of CAD can potentially prevent detrimental consequences such as myocardial infarction and congestive heart failure, which have significant implications in terms of societal financial burdens.
Currently, stress testing usually comes in one of several forms: treadmill ECG stress test, stress echo test, single photo electron computed tomography (SPECT) stress test, MR stress perfusion test, or PET stress perfusion test. Cardiac PET perfusion imaging is considered the reference technique for quantitative myocardial perfusion, due to its ability to quantify absolute myocardial blood flow (MBF) and coronary flow reserve, relative lack of attenuation artifact, and superior image quality (4). The most common PET perfusion tracers include 13N-NH3, 15O-H2O, 82Rubidium, and 18F-Flurpiridaz.
MR perfusion stress test has been used more commonly in the last few years, and meta-analysis showed that it was at least non-inferior if not superior to SPECT or echo stress tests (5). The cardiac MR perfusion stress test offers several advantages over PET/CT, including no radiation, no blooming effect from calcium, superior soft-tissue contrast and functional assessment of the heart, and potentially better morphologic assessment of coronary plaques.
In turn, a simultaneous PET/MR stress test offers several potential advantages over individual PET/CT or MR stress tests. That includes motion and partial volume correction, improved prognostic stratification using both functional (MR) and metabolic (PET) information, internal validation between MR and PET stress perfusion findings, and reduced radiation when compared to PET/CT (6).
Dr. Woodard’s group at Washington University demonstrated the feasibility of a 13N-NH3 PET/MR stress test in 2015 (7). Fifteen patients with positive SPECT stress test underwent cardiac 13N-NH3 PET/MR perfusion stress test, and the PET/MR results were compared to coronary angiographic findings. In this small cohort of patients, the PET/MR stress test had a non-significant superior diagnostic accuracy when compared to SPECT (64% vs. 50%). Interestingly, an abbreviated PET/MR stress test, where only stress perfusion images were obtained, yielded a similar ability to diagnose CAD. The rationale is that MR LGE can serve as “rest” image because it can distinguish infarct from normal or ischemic myocardium. This approach may potentially be useful in improving the competitiveness of a PET/MR stress test by reducing in half the total scan time.
A case study also demonstrated the potential superiority of a cardiac PET/MR stress test (8). In this case study, a 72-year-old woman who had a SPECT stress test that demonstrated mixed infarct and ischemia underwent simultaneous 13N-NH3 PET/MR stress test. To the authors’ surprise, no LGE was found, which suggested that there was actually no myocardial infarction. Subsequent analysis of quantitative MBF by PET showed a flow pattern suggestive of myocardial steal. Consequently, the area that was initially believed to be infarct in SPECT was likely actually hibernating myocardium, as determined by simultaneous PET/MR imaging. In conclusion, PET/MR stress test (either with 13N-NH3 or other tracers) is a promising area that warrants further investigations. It is noteworthy of the potential limitations of a PET/MR stress test. For example, PET/MR has a smaller inner bore than PET/CT, and cannot accommodate larger patients. Patients with metal implants or with significant renal impairment may not undergo contrast MR examination. Consequently, patient selection for a PET/MR stress test will be more stringent than other existing stress tests, potentially limiting its more widespread use.
Imaging the myocardial infarct
Multiple original studies and review articles investigated and speculated the utility of simultaneous cardiac PET/MR in myocardial infarct imaging (9-15).
Much of this work came from investigators in University Hospital Essen. In one of the earliest studies on simultaneous cardiac PET/MR, Nensa et al. examined 20 patients with myocardial infarctions by simultaneous 18F-Fluorodeoxyglucose (18F-FDG) PET/MR (9). Fourteen of them had recent acute ST-elevation myocardial infarction, one had non-ST-elevation myocardial infarction, and five had chronic myocardial infarction. The authors found substantial agreement between the reduced 18F-FDG in PET with MR LGE images (κ=0.76), and between reduced 18F-FDG in PET and MR cine images (κ=0.78) in their ability to diagnose myocardial infarct. In a subgroup analysis, 18F-FDG tracer uptake was comparable between 18F-FDG PET/CT and 18F-FDG PET/MR, demonstrating feasibility of the PET/MR examination.
In a subsequent study, they shifted their attention to the areas around the acute myocardial infarction scar (the “at-risk” area) (10). Twenty-five patients who suffered from acute coronary occlusion and received interventional reperfusion underwent simultaneous cardiac 18F-FDG PET/MR. Areas at risk were estimated by MR using an endocardial surface area method, and by PET by reduced myocardial 18F-FDG uptake. These two methods yielded good correlation (r=0.7, P=0.001). They noted that the areas of reduced 18F-FDG tended to be larger than the infarct size marked by LGE, similar to what others have observed (10).
Another group (11) investigated the correlations between 18F-FDG PET uptake pattern, MR LGE, and MR T2 mapping, again in the context of acute myocardial infarction. Twenty-one patients with ST-elevation myocardial infarction underwent simultaneous 18F-FDG PET/MR examination. 18F-FDG and T2 mapping correlated well, while the areas marked by MR LGE tended to be smaller than those measured by the other two methods. The authors contended that these areas demarcated by MR LGE are likely non-salvageable myocardium, whereas areas with no LGE but reduced 18F-FDG uptake are likely “at-risk” salvageable myocardium. Indeed, the salvage ability was demonstrated when functional recovery of the “at-risk” areas were observed in follow-up MR cine imaging, whereas recovery was less likely in the infarct core.
In conclusion, simultaneous PET/MR myocardial infarct imaging showed promise in identifying infarct and at-risk myocardium. Questions remain as to how a comprehensive cardiac PET/MR examination provides additive value over existing imaging modalities. One consideration is that for the subgroup of patients who suffered substantial myocardial infarction and subsequently developed significant systolic heart failure, they are at higher risk for ventricular arrhythmia. Fibrosis and scar imaging by simultaneous PET/MR can potentially be investigated in these patients. The findings can be correlated with risk of arrhythmia to yield potential outcome assessments, which may lend insight into the assessment of sudden cardiac death risk and potentially guide patient selection of implantable cardiac defibrillator (ICD) implantation.
Salvage after myocardial infarction: stem cell therapy
After myocardial infarction, fibrosis takes place to replace the necrotic myocardium, and the heart undergoes dilatation to allow the thinned area of the heart to withstand pressure and volume load, in the process called ventricular remodeling. This, coupled with the loss of myocardial tissue and contractility, can lead to the development of heart failure. Stem cell transplant represents a potentially promising therapeutic salvage therapy for myocardial function. However, currently, there is insufficient evidence for a beneficial effect of stem cell therapy in patients who suffered from acute myocardial infarction. In a systematic review of 41 RCTs totaling 2,732 patients, stem cell therapy was found to have no clear benefit on morbidity, quality of life/performance, or left ventricular ejection fraction measured by MR (16).
There are currently no published simultaneous PET/MR studies on human stem cell therapy post myocardial infarction. There are, however, pre-clinical animal studies looking at co-registered PET/MR images following stem cell transplant (17,18). Stem cells were labelled with super-paramagnetic iron oxide (SPIO) and 18F-FDG for separate MR and PET tracing. These studies found that there was generally good agreement on the size and location of the engrafted stem cells shown by co-registered PET and MR images. Functional outcomes were not measured in these studies.
In conclusion, while the use of PET/MR to track cardiac stem cell engraftment is still not done clinically, it remains a viable noninvasive monitoring option post stem cell transplant to monitor the location and viability of the transplanted cells, and warrants further investigations.
Carotid atherosclerosis
In addition to CAD detection and myocardial infarction imaging, carotid atherosclerosis imaging is the next main area of cardiovascular PET/MR research. Stroke is a highly morbid condition, and carotid plaques are estimated to account for 15–20% of all ischemic strokes (19,20). The classical thinking is that strokes related to carotid artery disease are caused by vulnerable plaques, typically lesions that have thin or ruptured fibrous cap, large lipid-rich core or necrotic core, or intraplaque hemorrhage (21). Metabolically active macrophages mediate this inflammatory process, leading to plaque instability. However, luminal narrowing alone was not particularly predictive of stroke risk (22), and in one study, up to 40% of patients with ischemic stroke had no definitive stenotic carotid lesions (23). Furthermore, higher 18F-FDG uptake was found to be an independent predictor of stroke recurrence, regardless of degree of luminal stenosis (24). Therefore, emphasis has been made to identify high-risk vascular plaques with non-significant luminal obstruction. Given the robust capabilities of PET/MR to fuse high resolution anatomic and metabolic plaque properties, this represents a promising area of clinical application for early high-risk carotid atherosclerosis detection.
Various groups have investigated simultaneous carotid PET/MR imaging (25-30). Ripa et al. in 2013 reported comparison of PET/MR and PET/CT images of carotid arteries in six HIV-positive patients at increased risk for atherosclerosis (25). Quantitative comparison of 18F-FDG uptake revealed strong agreement between PET data acquired by PET/MR and PET/CT, despite differences in the methods of PET attenuation correction, reconstruction algorithm, and detector technology between 18F-FDG PET/CT and 18F-FDG PET/MR. This proof-of-principle study demonstrated feasibility of PET/MR in carotid atherosclerosis imaging. However, in this small cohort of patients, no hemodynamically significant atherosclerotic disease was found, and the comparison of PET/MR and PET/CT was done in healthy carotid artery segments.
In 2014, Dr. Woodard’s group at Washington University reported a study where 22 high risk patients underwent simultaneous carotid 18F-FDG PET/MR imaging (26). Lesions identified as lipid-pool lesions by MR were more likely to be metabolically active on 18F-FDG PET when compared to lesions with fibrous plaque composition, even in hemodynamically non-obstructive lesions.
Rischpler et al. published an article earlier this year, where they studied a group of 18 patients who suffered from cryptogenic ischemic stroke and possessed non-stenotic carotid atherosclerotic plaques (27). MR images showed that up to 40% of these patients had American Heart Association type VI complicated plaques in the ipsilateral carotid artery, versus 0% on the contralateral side. Higher 18F-FDG uptake was found in these plaques, showing correlation between PET and MR findings. This study demonstrated that metabolically active and anatomically complex pathologies can be identified and characterized by 18F-FDG PET/MR in hemodynamically non-significant carotid segments ipsilateral to their stroke.
The above studies utilized 18F-FDG for PET imaging. It is worth noting that other specialized PET tracers are being explored for atherosclerotic imaging, including 18F-galacto-RGD to target angiogenesis, 18F-sodium fluoride to target calcification, and 64Cu-DOTATATE to target macrophages. One study by Pedersen et al. reported 10 patients with significant carotid atherosclerosis underwent simultaneous 64Cu-DOTATATE PET/MR carotid imaging (30). PET signal uptake was shown to correlate significantly with CD-163 gene expression (by real time polymerase chain reaction). Thus, using targeted PET tracers, morphologic findings by MR can now be analyzed not just against inflammation (by 18F-FDG PET), but also against various genetic expression patterns, furthering our understanding of the disease pathogenesis and potentially advancing therapeutic decision makings.
In conclusion, we believe that simultaneous carotid PET/MR imaging is a promising area where 18F-FDG and other novel PET tracers may be used to combine with high resolution MR images to better characterize early carotid artery disease. Specific limitations to carotid simultaneous PET/MR include motion artifacts with head movement, radiation exposure, as well as 18F-FDG PET signal spillover and interference from adjacent metabolically active structures such as the neck musculatures. Cost-effectiveness and superiority of the PET/MR exam will also need to be demonstrated.
Hereditary, infiltrative and inflammatory cardiovascular diseases
Hypertrophic cardiomyopathy (HCM)
HCM is the most common hereditary cardiovascular disease, affecting approximately 1 in 500 individuals. Single-nucleotide mutations in at least 11 sarcomere-related genes are associated with this autosomal dominant disease. HCM is characterized by abnormal left ventricular hypertrophy and myocardial fibrosis, leading to dynamic left ventricular outflow tract obstruction, syncope, cardiac arrhythmia, stroke, heart failure and sudden death. Several risk factors, such as family history of sudden death, left ventricular wall thickness ≥30 mm, non-sustained ventricular tachycardia, and unexplained syncope, were investigated in their ability to predict sudden cardiac death and candidacy for ICD (31). However, due to variable expressivity and penetrance (age-related), these risk factors showed a low positive predictive value (31).
LGE MR can readily pick up intramyocardial fibrosis in HCM patients, and this was shown to be associated with arrhythmia and sudden death (32). 18F-FDG PET studies on HCM patients were much less common (33,34), and cardiac PET imaging was not investigated previously in any outcome studies.
To date, there is one publication (case study) that looked at simultaneous PET/MR evaluation of an HCM patient (35). A 25-year-old man with incidental Echocardiographic finding of non-obstructive HCM with a LV septal thickness of 30mm underwent simultaneous 18F-FDG PET/MR. PET demonstrated patchy 18F-FDG defects within the hypertrophic septum. MR demonstrated reduced MBF in the hypertrophic septum, and LGE MR showed overlap of contrast enhancement with the area of reduced 18F-FDG uptake. Together, PET/MR demonstrated good correlation between anatomic, functional and metabolic impairments.
Nevertheless, despite good PET and MR correlation, there is currently no evidence to suggest superiority in using simultaneous PET/MR for HCM assessment. In patients with renal impairment prohibiting Gadolinium contrast MR study, simultaneous 18F-FDG PET/MR may be considered to assist in speculating the size and extent of fibrosis.
Anderson-fabry disease (AFD)
AFD is a rare X-linked lysosomal storage disease that is known to have potential cardiac involvement. Cardiac manifestations including arrhythmias, accelerated CAD, valvulopathy and heart failure, and may occur in up to 60% of all patients (36). Prior cardiac MR studies showed a characteristic basal inferolateral wall LGE pattern (37), and prolonged T2 relaxation time (38). On the other hand, existing cardiac PET studies had mostly looked at coronary microvascular dysfunction by quantitative MBF assessment in AFD patients (39).
There is evidence that early treatment with enzyme replacement therapy improves cardiovascular outcome (40). Hence PET/MR presents a potentially useful surveillance tool for early detection of cardiac involvement in AFD patients.
There is one simultaneous cardiac 18F-FDG PET/MR study exploring early detection of cardiac involvement in AFD patients (41). In this study, 13 AFD patients with no known cardiac involvement and preserved left ventricular systolic function by echocardiogram underwent simultaneous cardiac 18F-FDG PET/MR. Six out of 13 patients had positive MR findings (LGE and positive short inversion time inversion recovery), whereas all patients demonstrated either homogenously or heterogeneously increased 18F-FDG uptake. No outcome data were acquired in this study. Extrapolating from this limited data set, PET appeared to be more sensitive than MR in picking up early subclinical cardiac involvement, although both PET and MR were able to detect cardiac involvement earlier than echocardiography assessment of systolic dysfunction. Whether this early detection can translate into clinical benefit (with early enzyme replacement therapy) remains to be answered.
In conclusion, simultaneous PET/MR for AFD warrants further investigations. Given the potential reward in early detection and treatment of AFD, simultaneous cardiac PET/MR may be considered when feasible.
Amyloidosis
Amyloidosis PET/MR imaging is an interesting field that will likely receive increasing attention in the future. Cardiac amyloidosis frequently results from deposition of the immunoglobulin light chain (AL type) or the transthyretin (TTR type). Traditionally, diagnostic imaging of cardiac amyloidosis is done by Echocardiography or MR. On MR imaging there is a combination of characteristic findings including global subendocardial LGE, abnormal myocardial and blood-pool Gadolinium kinetics (difficulty optimizing TI during LGE), and severe concentric left ventricular hypertrophy and diastolic dysfunction (42).
There are recent advances in PET amyloid imaging with several tracers, including 11C-PiB (43), florbetapir (44), florbetaben, and flutemetamol (45). Whereas these tracers are formally indicated for neuroamyloid imaging for the diagnosis of Alzheimer’s disease, studies have shown that several of these tracers were detected in the myocardium in patients with systemic amyloid and suspected cardiac involvement (43-45).
To date, no studies were published investigating cardiac amyloid by simultaneous PET/MR. However, with the advances in plaque-specific PET tracers; we expect simultaneous PET/MR cardiac amyloid assessment studies to come in the near future. Potential benefits of a simultaneous PET/MR cardiac amyloid study include comprehensive survey of extracardiac amyloid involvements, added diagnostic accuracy (especially when Gadolinium cannot be administered due to renal impairment, which is not uncommon in patients with amyloid), and potentials for disease prognostication.
Cardiac sarcoidosis
Sarcoidosis is an inflammatory disease characterized by non-caseating granuloma formation in the lungs and mediastinal lymphadenopathy, with cardiac involvement in approximately 30–85% of cases (46). Most notable cardiac co-morbidities include atrioventricular conduction block and ventricular arrhythmia, sometimes necessitating pacemaker or ICD implantation. In cardiac sarcoidosis, the infiltrative fibrosis occurs in a patchy non-homogenous manner, rendering a high false negative rate in endomyocardial biopsy. Therefore, noninvasive imaging plays an important role in identifying potential cardiac sarcoidosis.
MR imaging is currently considered standard-of-care for the detection of cardiac sarcoidosis. It allows regional wall motion assessment (typical for cardiac sarcoidosis), cardiac edema assessment by T2 imaging, and LGE evaluation of fibrosis. The extent of cardiac edema seen on T2-weighted MR images may be used in longitudinal follow-up to guide immunosuppressive therapy or monitor disease progression (47).
There is also a well-established body of literature on the use of PET/CT imaging in cardiac sarcoidosis. 18F-FDG-PET/CT imaging was useful in disease prognostication (48), and reduction of 18F-FDG-PET tracer uptake was shown to correlate with improvement of AV conduction disease (49). Most cardiac sarcoidosis PET studies were done using the 18F-FDG tracer, which detects inflammation, but at least one study investigated the tracer of 13N-NH3, which detects tissue perfusion. Both tracers had similar sensitivity in detecting cardiac sarcoidosis (50), but 18F-FDG may be more sensitive than 13N-NH3 in monitoring response to steroid therapy (50). When compared to MR, PET also has the added benefit of a comprehensive whole-body assessment to detect or monitor extracardiac sarcoidosis.
In the pre-simultaneous PET/MR era, correlative studies of separate PET and MR images demonstrated comparable accuracy of both modalities in detecting myocardial lesions (51). In general, 18F-FDG PET detects active cardiac sarcoid disease, whereas LGE MR primarily detects more advanced fibrotic lesions. The active flare and chronic fibrotic changes represent two different stages of disease, which may overlap with each other, and distinguishing between these two phases may have therapeutic implications in terms of intensity of immunosuppressive therapy. Hence, a simultaneous cardiac PET/MR examination may be uniquely useful in providing a comprehensive assessment of the overall disease state.
To date, there is one publication (case study) examining cardiac sarcoidosis by simultaneous 18F-FDG PET/MR imaging (52). A 72-year-old woman with normal coronary arteries and mediastinal lymphadenopathy underwent simultaneous 18F-FDG PET/MR to assess the possibility of cardiac involvement (52). MR showed septal and inferoseptal hypokinesis by cine, lack of edema on T2-weighted MR images, and subepicardial LGE in the anteroseptum and inferoseptum. 18F-FDG PET showed decreased 18F-FDG uptake in the region of LGE, and increased 18F-FDG uptake around the fibrosis. Therefore, the combined 18F-FDG PET/MR data was able to identify both active and chronic disease in this particular case. Of note, T2-weighted MR sequences failed to detect tissue edema in areas with increased 18F-FDG uptake. This suggests that the combined 18F-FDG PET/MR may provide incremental value over standalone MR imaging.
In conclusion, cardiac sarcoidosis PET/MR imaging is a promising field, given its ability to characterize active vs. chronic disease, to identify extracardiac involvements, and to potentially guide longitudinal therapy response. One limitation is that many patients with sarcoidosis undergo ICD implantation for arrhythmia and sudden death prevention, rendering them unsuitable candidate for PET/MR imaging.
Myocarditis
In recent years, the use of cardiac MR has become more common in assisting the diagnosis of myocarditis. A subset of patients with myocarditis has a rapidly deteriorating clinical course, which warrants early investigation and aggressive intervention. The patchy nature of myocarditis means that endomyocardial biopsy may fail to make the diagnosis, and an accurate noninvasive imaging test would be most welcomed. On MR imaging there is characteristic epicardial LGE pattern (53). However, many patients with rapidly deteriorating myocarditis also suffer from acute renal failure from cardiorenal syndrome, prohibiting them from getting LGE MR imaging. Thus, 18F-FDG PET offers a potentially useful complement to MR in these situations.
Rischpler et al. showed a case study in his review article in 2015, demonstrating good correlation of myocardial segments with increased 18F-FDG uptake on PET and LGE on MR (14). The area of increased 18F-FDG PET uptake was much larger than MR LGE.
Since myocarditis often time has a self-limited clinical course, the addition of PET imaging to the standard MR exam may allow us to speculate whether the acute disease flare state is still ongoing, potentially offering guidance for the duration of aggressive medical therapy.
Vasculitis
One study examined 12 patients with known large vessel vasculitis and compared between 18F-FDG PET/MR and 18F-FDG PET/CT (54). SUVs obtained by PET/MR and PET/CT correlated well, demonstrating feasibility of the PET/MR exam. The addition of MR morphologic information to PET 18F-FDG uptake data led to an increased number of vessel segments (from 86 to 95 segments) being diagnosed to be vasculitic. The authors therefore concluded that simultaneous PET/MR exam for vasculitis is feasible, and holds promise for better determining the extent of disease involvement.
Cardiac mass
Malignancy in the heart is uncommon but potentially serious. Benign primary cardiac lesions include myxoma, lipoma, fibroelastoma, rhabdomyoma, etc., and malignant primary cardiac lesions include angiosarcoma, rhabdomyosarcoma, mesothelioma, fibrosarcoma, lymphoma, etc. Primary malignancy of the heart is much less common than metastatic diseases, which commonly arise from the lungs, breasts, renal cell cancer and melanoma. In addition, left ventricular thrombus is the most common non-tumor mass found during cardiac imaging. The clinical presentation of cardiac mass is variable, ranging from asymptomatic incidental findings, to debilitating symptoms such as heart failure and syncope, often depending on the location of the mass.
Multiple imaging modalities are currently used for cardiac mass imaging, including transthoracic echocardiography, transesophageal echocardiography, CT, MR and PET or PET/CT. Generally, transthoracic echocardiography and CT are the first line imaging modalities in detecting abnormal cardiac masses, owing to their widespread use. MR imaging is generally considered the gold standard (55,56): in a skilled center, a combination of cine, T1-weighted, T2-weighted, fat-suppression, first pass perfusion and LGE can assess the location, size, vascularity, and tissue characteristics of the cardiac mass. In one study, cardiac MR was noted to have a diagnostic accuracy of 92% (56). On the other hand, cardiac 18F-FDG PET/CT imaging of cardiac masses also carries a high diagnostic accuracy of 96% (57). Whole-body PET can be done more readily than whole-body MR due to scan time, and that allows for detection of extracardiac malignancy. To date, there is no specific PET tracer developed for detection of primary cardiac malignancy: 18F-FDG is the most widely used PET tracer for examining malignancy.
The argument for the use of PET/MR in cardiac mass imaging is that independent PET and MR data may provide reassurance that a lesion may be morphologically and metabolically benign, thus circumventing the risk of a potentially high-risk cardiac surgery.
To date, there is one publication on cardiac mass assessment by simultaneous 18F-FDG PET/MR imaging (58). Twenty patients with cardiac masses underwent integrated PET/MR imaging. Thirteen (13/20, 65%) were found to be malignant and seven (7/20, 35%) were benign. In this small cohort, there was 100% sensitivity and 92% specificity in determining tumor malignancy. However, since the standalone MR and PET already have high diagnostic accuracy, the incremental value of simultaneous PET/MR remains unclear.
In conclusion, because of already high performance of standalone PET/CT and MR imaging, cost concern and limited availability of PET/MR scanners, there is currently no strong argument for routine simultaneous PET/MR imaging in cardiac mass assessment. Simultaneous PET/MR may be considered in selected cases where incremental benefit can be expected.
Conclusions
In summary, in recent years, increasing attention was paid to the use of simultaneous PET/MR in cardiovascular imaging. Here we reviewed publications where simultaneous PET/MR was used to examine patients with various cardiovascular diseases, including CAD, myocardial infarction, carotid artery disease, sarcoidosis, amyloidosis, HCM, AFD, myocarditis, vasculitis, and cardiac masses. With General Electrics’ simultaneous Signa PET/MR system approved fairly recently in 2014 [Siemens’ Biograph mMR was the first one approved (2)], we anticipate further increase in both the overall PET/MR as well as cardiovascular-specific PET/MR publications in the years to come. Even though a “killer-application” has not been demonstrated for cardiovascular PET/MR, many areas, such as CAD detection, myocardial infarct imaging, and early carotid artery disease detection, show promise and warrant further investigations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Flotats A, Bravo PE, Fukushima K, Chaudhry MA, Merrill J, Bengel FM. 82Rb PET myocardial perfusion imaging is superior to 99mTc-labelled agent SPECT in patients with known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging 2012;39:1233-9. [Crossref] [PubMed]
- FDA clears new system to perform simultaneous PET, MRI scans. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258700.htm
- LaForest R, Woodard PK, Gropler RJ. Cardiovascular PET/MRI: Challenges and Opportunities. Cardiol Clin 2016;34:25-35. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Heart Disease Statistics and Maps. Available online: https://www.cdc.gov/heartdisease/facts.htm
- de Jong MC, Genders TS, van Geuns RJ, Moelker A, Hunink MG. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol 2012;22:1881-95. [Crossref] [PubMed]
- Nekolla SG, Martinez-Moeller A, Saraste A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging 2009;36 Suppl 1:S121-30. [Crossref] [PubMed]
- Durrani AK, Lau JM, Laforest R, Zheng J, Priatna A, Gropler RJ, Woodard PK. 13N-ammonia PET/MR Myocardial Stress Perfusion Imaging Early Experience. Radiological Society of North America 2015 Scientific Assembly and Annual Meeting. Available online: http://archive.rsna.org/2015/15012463.html
- Lau JM, Laforest R, Priatna A, Sharma S, Zheng J, Gropler RJ, Woodard PK. Demonstration of intermittent ischemia and stunning in hibernating myocardium. J Nucl Cardiol 2013;20:908-12. [Crossref] [PubMed]
- Nensa F, Poeppel TD, Beiderwellen K, Schelhorn J, Mahabadi AA, Erbel R, Heusch P, Nassenstein K, Bockisch A, Forsting M, Schlosser T. Hybrid PET/MR imaging of the heart: feasibility and initial results. Radiology 2013;268:366-73. [Crossref] [PubMed]
- Nensa F, Poeppel T, Tezgah E, Heusch P, Nassenstein K, Mahabadi AA, Forsting M, Bockisch A, Erbel R, Heusch G, Schlosser T. Integrated FDG PET/MR Imaging for the Assessment of Myocardial Salvage in Reperfused Acute Myocardial Infarction. Radiology 2015;276:400-7. [Crossref] [PubMed]
- Bulluck H, White SK, Fröhlich GM, Casson SG, O'Meara C, Newton A, Nicholas J, Weale P, Wan SM, Sirker A, Moon JC, Yellon DM, Groves A, Menezes L, Hausenloy DJ. Quantifying the Area at Risk in Reperfused ST-Segment-Elevation Myocardial Infarction Patients Using Hybrid Cardiac Positron Emission Tomography-Magnetic Resonance Imaging. Circ Cardiovasc Imaging 2016;9:e003900. [Crossref] [PubMed]
- Ratib O, Nkoulou R. Potential Applications of PET/MR Imaging in Cardiology. J Nucl Med 2014;55:40S-46S. [Crossref] [PubMed]
- Rakheja R, Chandarana H, Ponzo F, Seltzer AL, Beltran LS, Geppert C, Friedman KP. Fluorodeoxyglucose positron emission tomography/magnetic resonance imaging: current status, future aspects. PET Clin 2014;9:237-52. [Crossref] [PubMed]
- Rischpler C, Nekolla SG, Kunze KP, Schwaiger M. PET/MRI of the heart. Semin Nucl Med 2015;45:234-47. [Crossref] [PubMed]
- Naeger DM, Behr SC. PET/MR imaging: current and future applications for cardiovascular disease. Magn Reson Imaging Clin N Am 2015;23:95-103. [Crossref] [PubMed]
- Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2015.CD006536. [PubMed]
- Elhami E, Dietz B, Xiang B, Deng J, Wang F, Chi C, Goertzen AL, Mzengeza S, Freed D, Arora RC, Tian G. Assessment of three techniques for delivering stem cells to the heart using PET and MR imaging. EJNMMI Res 2013;3:72. [Crossref] [PubMed]
- Higuchi T, Anton M, Dumler K, Seidl S, Pelisek J, Saraste A, Welling A, Hofmann F, Oostendorp RA, Gansbacher B, Nekolla SG, Bengel FM, Botnar RM, Schwaiger M. Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med 2009;50:1088-94. [Crossref] [PubMed]
- Bonita R. Epidemiology of stroke. Lancet 1992;339:342-4. [Crossref] [PubMed]
- Risk of stroke in the distribution of an asymptomatic carotid artery. The European Carotid Surgery Trialists Collaborative Group. Lancet 1995;345:209-12. [Crossref] [PubMed]
- Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13-8. [Crossref] [PubMed]
- Yuan C, Mitsumori LM, Beach KW, Maravilla KR. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology 2001;221:285-99. [Crossref] [PubMed]
- Amarenco P. Underlying pathology of stroke of unknown cause (cryptogenic stroke). Cerebrovasc Dis 2009;27 Suppl 1:97-103. [Crossref] [PubMed]
- Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, Dolan E, Moroney J, Murphy S, O'Rourke K, O'Malley K, O'Donohoe M, McDonnell C, Noone I, Barry M, Crowe M, Kavanagh E, O'Connell M, Kelly PJ. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol 2012;71:709-18. [Crossref] [PubMed]
- Ripa RS, Knudsen A, Hag AM, Lebech AM, Loft A, Keller SH, Hansen AE, von Benzon E, Højgaard L, Kjær A. Feasibility of simultaneous PET/MR of the carotid artery: first clinical experience and comparison to PET/CT. Am J Nucl Med Mol Imaging 2013;3:361-71. [PubMed]
- Lau JM, Laforest R, Zheng J, Nie X, Priatna A, Woodard PK, Faul DD, Gropler RJ. 18F-FDG PET/MR Carotid Plaque Imaging: Early Experience. Radiological Society of North America 2014 Scientific Assembly and Annual Meeting. Available online: http://rsna2014.rsna.org/program/details/?emID=14004906
- Rischpler C, Nekolla SG, Beer AJ. PET/MR imaging of atherosclerosis: initial experience and outlook. Am J Nucl Med Mol Imaging 2013;3:393-6. [PubMed]
- Hyafil F, Schindler A, Sepp D, Obenhuber T, Bayer-Karpinska A, Boeckh-Behrens T, Höhn S, Hacker M, Nekolla SG, Rominger A, Dichgans M, Schwaiger M, Saam T, Poppert H. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging 2016;43:270-9. [Crossref] [PubMed]
- Vesey AT, Dweck MR, Fayad ZA. Utility of Combining PET and MR Imaging of Carotid Plaque. Neuroimaging Clin N Am 2016;26:55-68. [Crossref] [PubMed]
- Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Højgaard L, Ripa RS, Kjær A. 64Cu-DOTATATE PET/MRI for Detection of Activated Macrophages in Carotid Atherosclerotic Plaques: Studies in Patients Undergoing Endarterectomy. Arterioscler Thromb Vasc Biol 2015;35:1696-703. [Crossref] [PubMed]
- Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA 3rd, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007;298:405-12. [Crossref] [PubMed]
- Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 2010;3:51-8. [Crossref] [PubMed]
- Kuhn H, Gietzen FH, Schäfers M, Freick M, Gockel B, Strunk-Müller C, Jachmann E, Schober O. Changes in the left ventricular outflow tract after transcoronary ablation of septal hypertrophy (TASH) for hypertrophic obstructive cardiomyopathy as assessed by transoesophageal echocardiography and by measuring myocardial glucose utilization and perfusion. Eur Heart J 1999;20:1808-17. [Crossref] [PubMed]
- Funabashi N, Nakagawa K, Komuro I. Partial myocardial fibrosis in hypertrophic cardiomyopathy demonstrated by 18F-fluoro-deoxyglucose positron emission tomography and multislice computed tomography. Int J Cardiol 2006;107:284-6. [Crossref] [PubMed]
- Kong EJ, Lee SH, Cho IH. Myocardial Fibrosis in Hypertrophic Cardiomyopathy Demonstrated by Integrated Cardiac F-18 FDG PET/MR. Nucl Med Mol Imaging 2013;47:196-200. [Crossref] [PubMed]
- Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Ries M, Beck M. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 2004;34:236-42. [Crossref] [PubMed]
- Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 2003;24:2151-5. [Crossref] [PubMed]
- Imbriaco M, Spinelli L, Cuocolo A, Maurea S, Sica G, Quarantelli M, Pisani A, Liuzzi R, Cianciaruso B, Sabbatini M, Salvatore M. MRI characterization of myocardial tissue in patients with Fabry's disease. AJR Am J Roentgenol 2007;188:850-3. [Crossref] [PubMed]
- Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, Tome MT, McKenna WJ, Lee P, Camici PG. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 2006;92:357-60. [Crossref] [PubMed]
- Weidemann F, Sanchez-Niño MD, Politei J, Oliveira JP, Wanner C, Warnock DG, Ortiz A. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis 2013;8:116. [Crossref] [PubMed]
- Nappi C, Altiero M, Imbriaco M, Nicolai E, Giudice CA, Aiello M, Diomiaiuti CT, Pisani A, Spinelli L, Cuocolo A. First experience of simultaneous PET/MRI for the early detection of cardiac involvement in patients with Anderson-Fabry disease. Eur J Nucl Med Mol Imaging 2015;42:1025-31. [Crossref] [PubMed]
- Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111:186-93. [Crossref] [PubMed]
- Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjö L, Kero T, Långström B, Granstam SO, Rosengren S, Vedin O, Wassberg C, Wikström G, Westermark P, Sörensen J. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 2013;54:213-20. [Crossref] [PubMed]
- Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, Moore SC, Falk RH. Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 2014;41:1652-62. [Crossref] [PubMed]
- Lhommel R, Sempoux C, Ivanoiu A, Michaux L, Gerber B. Is 18F-flutemetamol PET/CT able to reveal cardiac amyloidosis? Clin Nucl Med 2014;39:747-9. [Crossref] [PubMed]
- Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest 1993;103:253-8. [Crossref] [PubMed]
- Vignaux O, Dhote R, Duboc D, Blanche P, Dusser D, Weber S, Legmann P. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002;122:1895-901. [Crossref] [PubMed]
- Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329-36. [Crossref] [PubMed]
- Takeda N, Yokoyama I, Hiroi Y, Sakata M, Harada T, Nakamura F, Murakawa Y, Nagai R. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002;105:1144-5. [Crossref] [PubMed]
- Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, Yoshikawa J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med 2003;44:1030-6. [PubMed]
- Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S, Tamaki N, Nishimura M. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008;35:933-41. [Crossref] [PubMed]
- White JA, Rajchl M, Butler J, Thompson RT, Prato FS, Wisenberg G. Active cardiac sarcoidosis: first clinical experience of simultaneous positron emission tomography--magnetic resonance imaging for the diagnosis of cardiac disease. Circulation 2013;127:e639-41. [Crossref] [PubMed]
- Sparrow PJ, Merchant N, Provost YL, Doyle DJ, Nguyen ET, Paul NS. CT. Radiographics 2009;29:805-23. [Crossref] [PubMed]
- Einspieler I, Thürmel K, Pyka T, Eiber M, Wolfram S, Moog P, Reeps C, Essler M. Imaging large vessel vasculitis with fully integrated PET/MRI: a pilot study. Eur J Nucl Med Mol Imaging 2015;42:1012-24. [Crossref] [PubMed]
- Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology 2013;268:26-43. [Crossref] [PubMed]
- Hoffmann U, Globits S, Schima W, Loewe C, Puig S, Oberhuber G, Frank H. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol 2003;92:890-5. [Crossref] [PubMed]
- Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T, Tiemann K, Maintz D, Scheld HH, Schober O, Weckesser M. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med 2012;53:856-63. [Crossref] [PubMed]
- Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Köhler J, Heusch P, Bruder O, Schlosser T, Nassenstein K. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med 2015;56:255-60. [Crossref] [PubMed]


