The role of cardiovascular magnetic resonance in the assessment of severe aortic stenosis and in post-procedural evaluation following transcatheter aortic valve implantation and surgical aortic valve replacement
Introduction
Aortic valve stenosis (AS) is the sequela of active valve remodelling which can readily be diagnosed but at present is beyond prevention. At the macroscopic level there is focal subendothelial thickening, inflammatory cell infiltration and subsequent calcification (1). There is therefore progressive narrowing of the aortic valve orifice leading to obstruction of left ventricular (LV) outflow with consequential myocardial hypertrophy to preserve wall stress and cardiac performance (2). Decompensation is driven by progressive myocyte death and myocardial fibrosis (3). Increased LV filling pressures and reduced cardiac output lead to exertional dyspnoea. Angina is also frequent from subendocardial ischaemia as a result of an increased LV mass and reduced coronary flow reserve (4). Severe aortic stenosis (AS) also carries an increased risk of sudden cardiac death (5).
Degenerative aortic valvular stenosis is the most common valve disease in the western world (6). The largest population-based study to date originates from the National Health, Lung and Blood Institute of 11,911 adults across the United States of America. Systematic echocardiographic examination indicated a prevalence ≤0.2% before 65 years of age, rising to 2.8% after 75 years (6). In 2010, there were an estimated 1.2 million people in the USA with at least moderate AS, including 520,000 aged over 75 years (7). The European Tromso study included 3,273 patients and reported higher prevalence in the elderly, affecting 9.8% of adults between 80 and 89 years of age. The annual incidence rate (derived from the study period 1974–2008) was 4.9 per 1,000 (8). Aging populations and the absence of any validated prevention method mean that the burden of AS is expected to double within the next 50 years (7).
The onset of symptoms is a major predictor of mortality; a concept first described by Ross and Braunwald in 1968 (9). The prognosis is particularly poor in the elderly (10) in whom there are other significant co-morbidities in more than one-third of cases (11). In octogenarians with comorbidities, mortality rates between 40% and 50% at 1 year have been reported (5). Five-year mortality has recently been reported at 60% after a first hospitalization with a diagnosis of AS (12). Two year follow-up data from the partner cohort B study (13) indicated standard medical treatment was associated with a cardiovascular mortality of 62.4% and repeat hospitalisation of 72.5%. Given the lack of effective medical treatment, management is centred on optimal timing of aortic valve intervention, to reverse hypertrophy, restore systolic and diastolic function, relieve symptoms and ultimately restore prognosis (14).
Aortic valve surgery
Surgical aortic valve replacement (SAVR) is a routine procedure that has been practised for over 50 years and its evidence base places it first-line in the treatment of symptomatic severe AS (15). The first-in-human heterotopic aortic valve replacement was performed in 1952 by Hufnagel and Harvey, palliating severe aortic regurgitation by implanting an artificial ball prosthesis in the descending aorta (16). In 1955, Murray placed a homograft in the same position (17). The advent of cardiopulmonary bypass facilitated maintenance of procedural haemodynamics and heralded the first sub-coronary mechanical AVR, performed by Starr and Harken in 1960. Two years later, Ross implanted a sub-coronary homograft (18). As a result, SAVR emerged as the gold standard for the management of AS. Crucial to the procedure is complete excision of calcified degenerated aortic cusps followed by precise implantation under direct vision of a modern xenograft or mechanical prosthesis using standard suturing techniques. Due to its ability to cure AS completely, conventional AVR has long since been considered the gold standard intervention (15).
Indeed, despite the intrusive nature, even elderly patients do favourably post-SAVR. In a cohort of over 1,000 octogenarians, survival rates of 89% and 69% after 1 and 5 years, respectively, were seen (19). Guidelines from Europe (20) and the USA (21) list a class I recommendation for SAVR in those with symptoms or reduced ejection fraction. However, surgery does carry an associated morbidity and mortality that may be considered prohibitive in elderly patients with multiple comorbidities and frailty. Indeed, in the Euro heart Survey, one third of 216 patients with symptomatic severe AS aged over 75 years were not referred on for surgery (22).
Transcatheter aortic valve implantation (TAVI)
The concept of a permanent “stent valve”, catheter-mounted, balloon-deployable valve prosthesis dates back over thirty years to animal experimental models (23). In 2002, the first-in-human TAVI was performed via an antegrade, transvenous approach (24). Later, the retrograde approach, with access via the femoral artery, gained favour and became a reproducible, fully percutaneous procedure (25). Since then, the rate of TAVI has risen enormously, with over 200,000 having been performed worldwide, the vast majority in Europe (26).
The early UK experience has been well charted through the construction of the UK TAVI registry (27). Data were collected prospectively on 870 patients until 31 December 2009. TAVI was performed with the use of the Medtronic CoreValve (Medtronic, Minneapolis, Minnesota, USA) (52%) or the Edwards-SAPIEN THV (Edwards Lifesciences, Irvine, California, USA) (48%). The majority of the TAVI implants (69%) were performed via the transfemoral approach according to the widespread ‘transfemoral first’ policy. Outcomes of TAVI patients in the UK TAVI Registry at 30 days, 1 year and 2 years were encouraging with mortality rates of 7.1%, 21.4% and 26.3%, respectively.
Health related quality of life measures are an important clinical outcome and are significantly improved following TAVI, with scores maintained out to 1 year (28); this is despite cerebral microinfarctions which are more frequently seen following TAVI than SAVR (29). In a cost-utility analysis, TAVI was demonstrated to be a cost-effective option in high-risk but operable elderly patients when compared with SAVR (30). TAVI improves survival and functional capacity when compared with standard medical therapy (10,31), and recently data suggests 2-year survival is superior to SAVR in high surgical risk patients (32) and similar at 5 years (33). Furthermore, the TAVI procedure is less restricted by patient frailty or confounding surgical considerations such as a “porcelain” aorta or mediastinal adhesions (34). Given its transformative benefits, TAVI is now an established intervention in symptomatic patients deemed inoperable or with too high a predicted postoperative mortality (35).
Cardiovascular magnetic resonance (CMR) and pre-procedure assessment
CMR imaging is a commonly used technique and determines both morphological and functional information that is crucial to the assessment of valvular heart disease. CMR permits high resolution imaging in any plane and can quantify the severity of the valvular lesions, determine aetiology, assess global and regional cardiac function as well as the anatomy of associated great vessels (36). Furthermore, myocardial perfusion, myocardial viability, tissue characterisation and proximal coronary anatomy can all be examined within a single study without any ionising radiation (37).
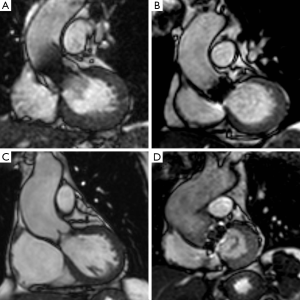
The typical CMR study for evaluating valvular heart disease comprises LV long-axis (2-, 3- and 4-chamber) views and a complete stack of sequential short-axis (every 8–10 mm from base to apex) cine images using a steady-state free precession (SFFP) pulse sequence (Figure 1). This generates images with an excellent signal-to-noise ratio and high blood-to-myocardium contrast, with a typical in-plane spatial resolution of (1.5–2.0 mm) comparable to transoesophageal echocardiography for aortic valve planimetry and assessment of cusp anatomy (38,39). CMR facilitates clear visualisation of sub-valvular and supra-valvular AS, and also permits assessment of prosthetic valvular function (Figure 2).
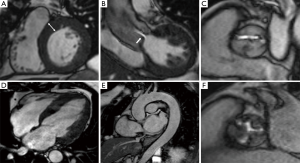
CMR is the most accurate technique for assessing both left and right ventricular volumes and mass (40-42). It has been validated against post-mortem studies of animal and human hearts (43) and is highly reproducible (44). Being 3-dimensional, it is also more sensitive to changes than one or two-dimensional measures (45) and independent of geometric assumptions of ventricular morphology. This can be crucial for the surveillance of asymptomatic patients to determine deterioration in ventricular function (36).
CMR permits direct flow quantification using through-plane phase contrast velocity mapping (46). This is a unique advantage of CMR which unlike echocardiography and invasive catheterisation, does not depend upon derivation from complex calculations (36). The technique measures phase shift of moving protons inside a magnetic field, exploiting their difference to stationary protons. This phase shift of moving protons is proportional to their velocity and velocity is measured after generating phase images (Figure 3) (46). However, the temporal resolution of CMR is typically 25–45 ms which is considerably lower than continuous wave Doppler echocardiography (which can be ~2 ms) (36). This in conjunction with turbulent flow artefacts and partial volume effects mean CMR peak velocity measurements may be underestimated compared to echocardiography, especially when peak velocities surpass 3.5–4.0 m/s (47).

Accurate measurements of the aortic root and ascending thoracic aorta can be ascertained (36) which may be dilated, particularly in context of bicuspid aortic valve disease, with important repercussions for subsequent surgical management. Furthermore, in patients with severe LV systolic dysfunction, a dobutamine-stress protocol may be employed to differentiate pseudo from true AS and determine contractile reserve (38).
The European Society of Cardiology guidelines for management of AS advocate CMR in particular for more detailed assessment in patients with paradoxical low-flow low-gradient AS, assessment of the ascending aorta when enlarged, and for the detection and quantification of myocardial fibrosis. This is in addition to assessment of ventricular volumes and systolic function (20). US guidelines similarly indicate CMR may be required to determine optimal treatment for a patient as an ancillary investigation to transthoracic echocardiography (21).
CMR detection of fibrosis and predicting prognosis
AS increases LV afterload and triggers an initial compensatory hypertrophic response. Women develop a concentrically hypertrophied, small cavity LV, whereas men are more prone to the development of eccentric hypertrophy (48). However, left untreated, there is progressive myocyte necrosis and subsequent replacement myocardial fibrosis (49). This is associated with abnormal cardiac remodelling and increased ventricular stiffness in both animal and human studies (50) and ultimately culminates in heart failure and a worse prognosis (51). Myocardial fibrosis has thus been targeted extensively as a potentially objective marker of LV decompensation that may hold promise in guiding appropriately timed valve intervention.
Historically, the reference standard for validating myocardial fibrosis has been myocardial biopsy but this is invasive, susceptible to sampling errors and does not assess the whole heart (50). There have been varying degrees of interstitial fibrosis reported on histological assessment in patients with severe AS, ranging from 4% to 39% (52,53).
A pivotal and unique strength of CMR is in vivo tissue characterisation, offering a direct visualization, whole-heart assessment of myocardial fibrosis (54) (Figure 4). The technique probes the retention of gadolinium-based contrast agents within myocardial tissue, with dead or scarred myocardium appearing bright in contrast to normal black myocardium on late inversion recovery T1-weighted imaging (55). The use of late gadolinium enhancement (LGE) imaging has been validated against surgical biopsy studies in AS (56), with focal mid-wall enhancement reportedly present in 19–62% of patients (51) and with increasing quantities seen with increasing hypertrophy (57).
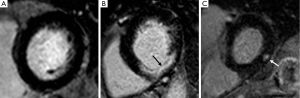
The degree of myocardial fibrosis at histology correlates with worsening NYHA class and impaired longitudinal systolic function, and is inversely associated with the degree of functional improvement following SAVR (58). In another histology study, fibrosis quantity was strongly associated with increased LV cavity diameters, and reduced LV ejection fraction; a finding also demonstrated from CMR imaging (59). Furthermore, pre-operative fibrosis grade was the strongest independent predictor of mortality post AVR (60).
Following on from biopsy observations, LGE imaging has been used to assess the clinical significance of fibrosis in patients with severe AS, both prior to and after valve intervention. The presence of mid-wall fibrosis in this context is associated with raised plasma troponin concentrations (61) and a hypertrophic strain pattern on electrocardiogram tracing (62), both of which can provide incremental prognostic information in asymptomatic patients. In a small cohort of patients (n=52, including 24 with aortic regurgitation) the quantity of fibrosis was a multivariate predictor of all-cause mortality and, in a subset of these patients, predicted lack of improvement of ejection fraction after SAVR (56). Another study reported that the absence of fibrosis was associated with good prognosis after SAVR for AS and that the extent of LGE did not change after SAVR (58).
In a larger study of 143 medically treated patients (40% moderate, 60% severe AS), presence of mid-wall hyperenhancement was associated with an 8-fold increase in all-cause mortality in comparison to patients without fibrosis, despite comparable valvular haemodynamics. Half the study population eventually underwent SAVR, and in this group the mortality rate was 53.8 per 1,000 patient years in those with mid-wall fibrosis, compared with 13.7 in those without focal fibrosis (63). In a subsequent publication, the incidence of major adverse cardiac events (MACE), stroke and heart block following SAVR were significantly higher in those with mid-wall fibrosis compared to those without. There were no 30-day MACE events, nor patient deaths at 2-year follow-up in those without fibrosis, highlighting the potential use of CMR in predicting risk/outcome prior to AVR for AS (64).
The largest study to date investigating the prognostic importance of CMR defined focal fibrosis involved 194 consecutive patients, all with severe AS undergoing SAVR (n=154) and TAVI (n=40) (65). This study demonstrated the presence and extent of myocardial fibrosis detected by CMR imaging predicted increased perioperative risk and worse all-cause mortality in those undergoing SAVR, and increased cardiovascular related mortality in both those undergoing SAVR and TAVI. Furthermore, the authors observed a high incidence of sudden cardiac death in those with fibrosis raising the possibility that prophylactic implantable cardioverter-defibrillators may improve long-term survival.
The evidence thus far indicates fibrosis detection using CMR heralds LV decompensation and there are on-going prospective studies to confirm whether this technique holds prognostic importance and could potentially improve patient selection for intervention (https://ClinicalTrials.gov: PRIMID-AS, RELIEF-AS, and NCT01755936).
Myocardial perfusion reserve (MPR)
The MPR is derived as the ratio of myocardial blood flow during maximal hyperaemia compared to resting conditions (66). In the absence of epicardial disease, it therefore indicates the presence of coronary microvascular dysfunction (67). MPR can be measured using CMR and has been shown in one study to independently predict aerobic exercise capacity in 46 patients with severe AS; with a strong inverse relationship to symptom status (68). However, CMR quantification of MPR is complicated and lacks consensus (55). The recently completed PRIMID-AS trial was designed to compare CMR with exercise testing in identifying patients likely to benefit from SAVR, and thus will help clarify the role of CMR MPR in AS (69).
Assessment of aortic stiffness
Aortic function regulates the entire cardiovascular system and changes in aortic wall composition and elasticity are important to the development of cardiovascular disease. Increased arterial stiffness is an independent predictor of adverse outcomes in patients with hypertension, renal failure, diabetes and the elderly (70) and is thus increasingly a clinical focus. CMR permits the measurement of both aortic distensibility (reflecting the systolic expansion of the aorta) and pulse wave velocity (the propagation speed of the pressure wave along the length of the aorta). CMR holds several advantages over conventional ultrasound, but most notably can reproducibly detect more subtle changes in regional stiffness at any operator chosen location (71). CMR has been used to study patients with bicuspid aortic valve disease, in whom significantly reduced elasticity of the entire thoracic aorta is observed, even without significant stenosis (72).
CMR and post-intervention assessment
Detection of myocardial injury
CMR is the gold standard imaging technique for the non-invasive detection and quantification of myocardial infarction (73), and has been used to investigate myocardial injury following treatment for severe AS (74,75). Using LGE CMR, focal fibrosis due to prior myocardial infarction is typically subendocardial in distribution, extending transmurally towards the epicardium the larger the infarct, and confined to a specific epicardial coronary artery territory; a pattern entirely distinct from that of mid-wall myocardial fibrosis (76).
In a CMR study of 50 patients (25 SAVR, 25 TAVI); new postoperative sub-endocardial infarction was evident in six individuals (5 SAVR, 1 TAVI, P=0.11). Despite the small numbers, the study was the first to suggest TAVI expansion was not detrimental to the patency of coronary ostia; and that perioperative myocardial protection in severely hypertrophied ventricles could, on occasion, be suboptimal during SAVR (74). In a larger study of patients undergoing TAVI for severe AS (n=61), new myocardial late enhancement with an ischaemic pattern occurred in 18%; averaging 1.8% of the LV mass in quantity. This was assumed to be embolic in origin, but importantly, did not correlate with cardiac biomarkers of injury, which were ubiquitously elevated in all patients. Furthermore, patients with injury detectable by CMR had a significant reduction in LV function at discharge (75). Further work is needed to evaluate the prognostic significance of CMR detected new myocardial infarction following TAVI, as has been done with elevated serum biomarkers (77).
Reverse ventricular remodelling
AS increases the afterload of the LV which compensates through alteration in wall geometry to preserve wall stress. LV hypertrophy is part of this pathophysiological adaptation and a remodelling process is well recognised comprising myocyte degeneration, replacement fibrosis and reduced ventricular performance. SAVR restores valvular function and a subsequent “reverse remodelling” ensues with mass regression, volumetric reduction and improved function. Indeed, this reverse remodelling underscores the improvement of symptoms and prognosis conferred by SAVR (74).
CMR affords greater precision to 2D echocardiography in the quantification of LV volumes and mass without the requirement for geometric assumptions, and has been used to characterise reverse ventricular remodelling in detail following both SAVR (74) and TAVI (74,78). In a study of 50 patients (25 SAVR, 25 TAVI) CMR was used to directly compare changes between baseline and 6 months following intervention (74). Both TAVI and SAVR were associated with significant and comparable reduction in the LV end systolic volume and LV mass index, with a greater reduction in LV end diastolic volume seen following SAVR compared to post-TAVI. Overall, adjusting for baseline characteristics, the authors felt global geometric reverse remodelling was unlikely to differ between the two procedures.
Interestingly, right ventricular reverse remodelling seemed more favourable following TAVI with a reduction in volumes and improved function observed. This was in contrast to SAVR, where a decline in RV function was reported; likely reflecting adverse effects of cardiopulmonary bypass during cardiac surgery. In this study, the presence of myocardial scar due to infarction, and not focal myocardial fibrosis, was associated with worse right ventricular function and volumes at 6 months. Statistically, worse baseline measures of LV volumes and mass were independent predictors of reduced reverse remodelling (defined as the LV mass:EDV ratio). These findings again highlight the potential importance of CMR in predicting patient outcomes and those likely to benefit from closer clinical observation.
Quantification of aortic regurgitation following TAVI
The TAVI procedure involves destruction of the native aortic valve leaflets, which are crushed by a superimposed bioprosthesis as it is expanded within the aortic annulus. Extensive native valve leaflet calcification, patient/prosthesis mismatch, under expansion of TAVI prosthesis and malposition can preclude a complete sealing of the paravalvular space with resultant paravalvular aortic regurgitation (PAR) (79). Furthermore, the two frequently used TAVI designs, namely the Medtronic CoreValve and the Edwards SAPIEN, comprise a skirt that covers only the lower part of the TAVI frame, leaving the upper part exposed. The term “supra-skirtal regurgitation” describes leakage through the uncovered part of the prosthesis above the skirt that may occur if the prosthesis is implanted too low in the aortic position (80).
A number of trials and multicentre registries have published data on PAR with an overall incidence ranging between 50% and 85% (79). A recent meta-analysis including 12,926 TAVI patients reported a pooled estimate incidence of moderate or severe PAR of 11.7% (81).
The significance of PAR post TAVI is in prognostication. Moderate to severe AR is an independent predictor of mortality in the postoperative period to 30 days, at 1 year, and at 2 years (79). In a recent study of 2,434 patients, the largest single study published, 1 year all-cause mortality, cardiac related mortality and rehospitalisation were significantly increased with worsening PAR. The presence of both mild (hazard ratio 1.27) and moderate-severe PAR (hazard ratio 2.18) were independently associated with higher late mortality on multivariate analysis (82).
The difference in rates of PAR reported after TAVI undoubtedly arises from the variety of imaging methods, time points and grading scales applied to the particular cohort. In clinical practise, 2D transthoracic echocardiography is the most frequently used modality to evaluate PAR severity given its low cost and availability. However, 2D echocardiography is by its nature largely qualitative and suited to central regurgitation; with image quality susceptible to body habitus, prior cardiac surgery or airway disease impeding acoustic windows (83).
A semi-quantitative assessment is possible but has considerable limitations when applied to eccentric and multiple jets arising from a crescentic irregular orifice, typically seen in the TAVI patient. The Valve Academic Research Consortium (VARC) has defined quantification criteria to improve uniformity in assessment of PAR post-TAVI. However, the use of the grading scheme for native valve regurgitation in this post-TAVI setting has not been validated (79).
CMR affords a number of advantages over echocardiography for the assessment of PAR. It permits full quantitation of regurgitant volumes irrespective of valve type, jet number or eccentricity and is unaffected by calcification or prosthesis artefact (84). Furthermore, a comprehensive evaluation of the consequences of PAR upon LV volumes and function can be determined concomitantly. Indeed the use of CMR to assess both valvular and ventricular function in the post-TAVI setting has been validated (83).
CMR is susceptible to arrhythmia and motion artefact and the final regurgitant volume assessment will include diastolic coronary flow (84). Nonetheless, in a recent comparison applying VARC-2 recommendations of 2D, 3D echocardiography and CMR in 71 patients, the intra- and inter-observer variability in determining regurgitant volume was found to be lowest with CMR (2.2%±2.0% and 1.5%±1.5% respectively) (85). In another recent comparison of quantitative CMR with 2D echocardiography, 27 of 56 (48%) TAVI patients had AR which was at least one grade more severe on CMR than echo indicating echo underestimates the degree of PAR (83). This may in part explain why even patients with reportedly “mild” PAR from PARTNER exhibited increased mortality (13). Further work is required to determine whether CMR is indeed of superior prognostic value in patients following TAVI.
Assessment of myocardial deformation and strain imaging
Quantification of myocardial strain and strain rate permits a distinct functional assessment of the radial, longitudinal and circumferential fibres of the LV and can detect contractile dysfunction prior to an overall decline in ejection fraction. Strain imaging has demonstrated prognostic importance in a number of cardiac conditions (86). Myocardial tissue tagging using CMR was first introduced in 1988 and remains the current gold standard CMR method to assess strain with proven reproducibility (87). This technique has been used in patients with symptomatic severe AS in whom pressure overload induces increased systolic wringing motion (thought to be compensatory) that progressively declines as hypertrophy and dilatation worsens. Following SAVR, there is normalisation of LV torsion (88), but interestingly, this disproportionately favours those without coronary disease (89).
Feature tracking is a novel technique involving more rapid semi-automatic analysis of standard CMR cine images. It has been compared to tissue tagging in patients with AS and consistently produces higher values with excellent reproducibility (86). It can detect subtle LV impairment not visible in standard echocardiography and has been used to assess LV performance in patients undergoing TAVI, in whom a trans-apical approach results in significant apical LV dysfunction when compared to a trans-femoral TAVI (90).
Future applications
CMR spectroscopy
Myocardial triglyceride content can be quantified using 1H CMR spectroscopy, and a number of studies have reported an independent correlation between degree of myocardial steatosis and both systolic and diastolic dysfunction (91,92). This technique has been used to demonstrate the presence of myocardial steatosis in patients with severe AS, both with and without symptoms. Myocardial triglyceride content, validated against histological quantification, was independently associated with degree of LV systolic strain impairment, despite a normal ejection fraction. Furthermore, steatosis and strain impairment were reversible following SAVR (93). Excessive fatty acids are precursors to toxic intermediates that promote apoptosis and ultimately change myocardial architecture (94). Myocardial lipotoxicity is thus a potentially treatable target which could offset LV dysfunction in AS and signal a role for CMR spectroscopy in risk stratification.
4D flow imaging
Two-dimensional phase-contrast CMR imaging has been used for over three decades to evaluate pulsatile blood flow of the heart and great vessels (95). Further advances in technology have heralded phase-contrast with flow-encoding in all three spatial directions that is resolved relative to all three dimensions of space, and to the dimension of time along the cardiac cycle (3D + time = 4D); referred to as “4D flow CMR” (96). This provides full volumetric coverage of any cardiac or vascular region of interest; with subsequent post hoc analysis used to quantify total flow, peak velocity or regurgitant fraction amongst other parameters (Figure 5) (97). Furthermore, deriving advanced haemodynamics such as wall shear stress (98), pressure difference (99) and turbulent kinetic energy (100) may facilitate unprecedented assessment of cardiovascular disease beyond simple flow measures (95).
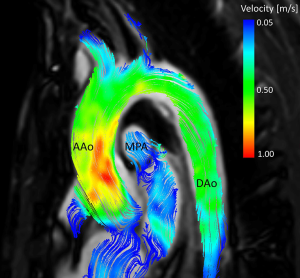
Bicuspid aortic valve disease is associated with an aortopathy and carries a risk of aortic dissection. Aortic dimensions are the principal measurement to guide intervention currently, given no measures of AS have proven useful in risk stratification (21). 4D flow CMR has offered unique insights into this aortopathy which is an area of significant clinical interest (95). In an assessment of 30 patients with bicuspid aortic valve [n=15 right-left phenotype (BAV-RL), n=15, right-non phenotype (BAV-RN)], 4D CMR flow indicated differences in aortopathy expression (101). In comparison to controls, the BAV-RL valve had elevated wall shear stress at the right-anterior wall with aortic enlargement predominantly affecting the tubular portion of the ascending aorta; in contrast to the BAV-RN valve which affected the right-posterior wall with dilatation affecting either the root only or the entire ascending aorta and arch. This unique assessment of haemodynamics with 4D flow CMR indicates a physiological mechanism through which bicuspid morphology may impact on aortopathy phenotype.
4D flow CMR has also been used to assess aortic flow following intervention for AS. Rather than physiologic central flow, all stented, stentless and mechanical SAVR prostheses showed eccentric flow jets mainly directed towards the right-anterior aortic wall, with significantly increased local wall shear stress where the flow jet impinged on the aorta (102). Furthermore, aortic blood flow following SAVR and TAVI have been directly compared, with both interventions producing similar asymmetric distributions of wall shear stress, but SAVR triggering more extensive vertical and helical (turbulent) flow patterns (103).
Conclusions
CMR is a well-established imaging technique that is non-invasive and devoid of ionising radiation, offering incremental value in the assessment of patients with AS, both prior to and after valve intervention. In a single imaging session, CMR can provide detailed information on cardiac and aortic anatomy, ventricular volumes and mass, myocardial tissue characterisation and valvular morphology and function; both native and prosthetic. There is a growing body of evidence that CMR can predict clinical outcomes in patients undergoing therapy for severe AS and ongoing clinical trials are likely to underscore the importance of CMR in managing this common and high-risk cardiac condition.
Acknowledgements
We are grateful to Dr. Pankaj Garg for his assistance in producing Figure 5.
Funding: JP Greenwood and S Plein received a research grant from Philips Healthcare. JP Greenwood is funded by a British Heart Foundation Project Grant (PG/11/126/29321). S Plein is funded by a British Heart Foundation Senior Fellowship (FS/10/62/28409).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011;124:1783-91. [Crossref] [PubMed]
- Clayton B, Morgan-Hughes G, Roobottom C. Transcatheter aortic valve insertion (TAVI): a review. Br J Radiol 2014;87:20130595. [Crossref] [PubMed]
- Chin CW, Vassiliou V, Jenkins WS, Prasad SK, Newby DE, Dweck MR. Markers of left ventricular decompensation in aortic stenosis. Expert Review of Cardiovascular Therapy 2014;12:901-12. [Crossref] [PubMed]
- Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation 1997;95:892-8. [Crossref] [PubMed]
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30:962-70. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Iung B, Vahanian A. Degenerative calcific aortic stenosis: a natural history. Heart 2012;98 Suppl 4:iv7-13. [Crossref] [PubMed]
- Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromso study. Heart 2013;99:396-400. [Crossref] [PubMed]
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Iung B, Baron G, Tornos P, Gohlke-Barwolf C, Butchart EG, Vahanian A. Valvular heart disease in the community: a European experience. Curr Probl Cardiol 2007;32:609-61. [Crossref] [PubMed]
- Berry C, Lloyd SM, Wang Y, Macdonald A, Ford I. The changing course of aortic valve disease in Scotland: temporal trends in hospitalizations and mortality and prognostic importance of aortic stenosis. Eur Heart J 2013;34:1538-47. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB; PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis 2014;56:565-71. [Crossref] [PubMed]
- Walther T, Blumenstein J, van Linden A, Kempfert J. Contemporary management of aortic stenosis: surgical aortic valve replacement remains the gold standard. Heart 2012;98 Suppl 4:iv23-9. [Crossref] [PubMed]
- Hufnagel CA, Harvey WP. The surgical correction of aortic regurgitation preliminary report. Bull Georgetown Univ Med Cent 1953;6:60-1. [PubMed]
- Murray G. Homologous aortic-valve-segment transplants as surgical treatment for aortic and mitral insufficiency. Angiology 1956;7:466-71. [Crossref] [PubMed]
- Ross DN. Homograft replacement of the aortic valve. Lancet 1962;2:487. [Crossref] [PubMed]
- Asimakopoulos G, Edwards MB, Taylor KM. Aortic valve replacement in patients 80 years of age and older: survival and cause of death based on 1100 cases: collective results from the UK Heart Valve Registry. Circulation 1997;96:3403-8. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Sch?fers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P. Gohlke-B?rwolf C, Boersma E, Ravaud P, Vahanian A. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714-20. [Crossref] [PubMed]
- Binder RK, Webb JG. TAVI: from home-made prosthesis to global interventional phenomenon. Heart 2012;98 Suppl 4:iv30-6. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006;113:842-50. [Crossref] [PubMed]
- Newton JD, Redwood S, Prendergast BD. Transcatheter aortic valve implantation: a durable treatment option in aortic stenosis? Heart 2015;101:913-4. [Crossref] [PubMed]
- Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8. [Crossref] [PubMed]
- Fairbairn TA, Meads DM, Mather AN, Motwani M, Pavitt S, Plein S, Blackman DJ, Greenwood JP. Serial change in health-related quality of life over 1 year after transcatheter aortic valve implantation: predictors of health outcomes. J Am Coll Cardiol 2012;59:1672-80. [Crossref] [PubMed]
- Uddin A, Fairbairn TA, Djoukhader IK, Igra M, Kidambi A, Motwani M, Motwani M, Herzog B, Ripley DP, Musa TA, Goddard AJ, Blackman DJ, Plein S, Greenwood JP. Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement: effect on health-related quality of life. Circ Cardiovasc Interv 2015;8:e001913. [Crossref] [PubMed]
- Fairbairn TA, Meads DM, Hulme C, Mather AN, Plein S, Blackman DJ, Greenwood JP. The cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at high operative risk. Heart 2013;99:914-20. [Crossref] [PubMed]
- Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR. PARTNER trial investigators. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2485-91. [Crossref] [PubMed]
- Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB Jr, Chetcuti S, Heiser J, Merhi W, Zorn GL 3rd, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte JV, Resar JR, Aharonian V, Pfeffer T, Oh JK, Qiao H, Popma JJ. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113-21. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG. PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellière R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90. [Crossref] [PubMed]
- Sorajja P, Pedersen W. Next-generation transcatheter aortic valve replacement: evolution of a revolution. J Am Coll Cardiol 2014;64:1349-51. [Crossref] [PubMed]
- Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:7. [Crossref] [PubMed]
- Ripley DP, Motwani M, Plein S, Greenwood JP. Established and emerging cardiovascular magnetic resonance techniques for the assessment of stable coronary heart disease and acute coronary syndromes. Quant Imaging Med Surg 2014;4:330-44. [PubMed]
- Lopez-Mattei JC, Shah DJ. The role of cardiac magnetic resonance in valvular heart disease. Methodist Debakey Cardiovasc J 2013;9:142-8. [Crossref] [PubMed]
- Paelinck BP, Van Herck PL, Rodrigus I, Claeys MJ, Laborde JC, Parizel PM, Vrints CJ, Bosmans JM. Comparison of magnetic resonance imaging of aortic valve stenosis and aortic root to multimodality imaging for selection of transcatheter aortic valve implantation candidates. Am J Cardiol 2011;108:92-8. [Crossref] [PubMed]
- Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 2000;21:1387-96. [Crossref] [PubMed]
- Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension 2002;39:750-5. [Crossref] [PubMed]
- Koch JA, Poll LW, Godehardt E, Korbmacher B, Mödder U. Right and left ventricular volume measurements in an animal heart model in vitro: first experiences with cardiac MRI at 1.0 T. Eur Radiol 2000;10:455-8. [Crossref] [PubMed]
- Childs H, Ma L, Ma M, Clarke J, Cocker M, Green J, Strohm O, Friedrich MG. Comparison of long and short axis quantification of left ventricular volume parameters by cardiovascular magnetic resonance, with ex-vivo validation. J Cardiovasc Magn Reson 2011;13:40. [Crossref] [PubMed]
- Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29-34. [Crossref] [PubMed]
- Myerson SG, Montgomery HE, World MJ, Pennell DJ. Left ventricular mass: reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension 2002;40:673-8. [Crossref] [PubMed]
- Gatehouse PD, Keegan J, Crowe LA, Masood S, Mohiaddin RH, Kreitner KF, Firmin DN. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol 2005;15:2172-84. [Crossref] [PubMed]
- O'Brien KR, Cowan BR, Jain M, Stewart RA, Kerr AJ, Young AA. MRI phase contrast velocity and flow errors in turbulent stenotic jets. J Magn Reson Imaging 2008;28:210-8. [Crossref] [PubMed]
- Dobson LE, Fairbairn TA, Plein S, Greenwood JP. Sex Differences in Aortic Stenosis and Outcome Following Surgical and Transcatheter Aortic Valve Replacement. J Womens Health (Larchmt) 2015;24:986-95. [Crossref] [PubMed]
- Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003;107:984-91. [Crossref] [PubMed]
- Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57:891-903. [Crossref] [PubMed]
- Chin CW, Pawade TA, Newby DE, Dweck MR. Risk Stratification in Patients With Aortic Stenosis Using Novel Imaging Approaches. Circ Cardiovasc Imaging 2015;8:e003421. [Crossref] [PubMed]
- Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989;79:744-55. [Crossref] [PubMed]
- Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010;122:138-44. [Crossref] [PubMed]
- Ambale-Venkatesh B, Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol 2015;12:18-29. [Crossref] [PubMed]
- Singh A, Steadman CD, McCann GP. Advances in the understanding of the pathophysiology and management of aortic stenosis: role of novel imaging techniques. Can J Cardiol 2014;30:994-1003. [Crossref] [PubMed]
- Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278-87. [Crossref] [PubMed]
- Debl K, Djavidani B, Buchner S, Lipke C, Nitz W, Feuerbach S, Riegger G, Luchner A. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart 2006;92:1447-51. [Crossref] [PubMed]
- Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M. Gattenl?hner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577-84. [Crossref] [PubMed]
- Nigri M, Azevedo CF, Rochitte CE, Schraibman V, Tarasoutchi F, Pommerantzeff PM, Brandão CM, Sampaio RO, Parga JR, Avila LF, Spina GS, Grinberg M. Contrast-enhanced magnetic resonance imaging identifies focal regions of intramyocardial fibrosis in patients with severe aortic valve disease: Correlation with quantitative histopathology. Am Heart J 2009;157:361-8. [Crossref] [PubMed]
- Milano AD, Faggian G, Dodonov M, Golia G, Tomezzoli A, Bortolotti U, Mazzucco A. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg 2012;144:830-7. [Crossref] [PubMed]
- Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J 2014;35:2312-21. [Crossref] [PubMed]
- Shah AS, Chin CW, Vassiliou V, Cowell SJ, Doris M, Kwok TC, Semple S, Zamvar V, White AC, McKillop G, Boon NA, Prasad SK, Mills NL, Newby DE, Dweck MR. Left ventricular hypertrophy with strain and aortic stenosis. Circulation 2014;130:1607-16. [Crossref] [PubMed]
- Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271-9. [Crossref] [PubMed]
- Quarto C, Dweck MR, Murigu T, Joshi S, Melina G, Angeloni E, Prasad SK, Pepper JR. Late gadolinium enhancement as a potential marker of increased perioperative risk in aortic valve replacement. Interact Cardiovasc Thorac Surg 2012;15:45-50. [Crossref] [PubMed]
- Barone-Rochette G, Pierard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, Pouleur AC, Vancraeynest D, Pasquet A, Vanoverschelde JL, Gerber BL. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol 2014;64:144-54. [Crossref] [PubMed]
- Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, Pibarot P. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985) 2009;106:113-21. [PubMed]
- Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation 2003;107:3170-5. [Crossref] [PubMed]
- Steadman CD, Jerosch-Herold M, Grundy B, Rafelt S, Ng LL, Squire IB, Samani NJ, McCann GP. Determinants and functional significance of myocardial perfusion reserve in severe aortic stenosis. JACC Cardiovasc Imaging 2012;5:182-9. [Crossref] [PubMed]
- Singh A, Ford I, Greenwood JP, Khan JN, Uddin A, Berry C, Neubauer S, Prendergast B, Jerosch-Herold M, Williams B, Samani NJ, McCann GP. Rationale and design of the PRognostic Importance of MIcrovascular Dysfunction in asymptomatic patients with Aortic Stenosis (PRIMID-AS): a multicentre observational study with blinded investigations. BMJ Open 2013;3:e004348. [Crossref] [PubMed]
- Ripley DP, Negrou K, Oliver JJ, Worthy G, Struthers AD, Plein S, Greenwood JP. Aortic remodelling following the treatment and regression of hypertensive left ventricular hypertrophy: a cardiovascular magnetic resonance study. Clin Exp Hypertens 2015;37:308-16. [Crossref] [PubMed]
- Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 2011;57:1511-22. [Crossref] [PubMed]
- Grotenhuis HB, Ottenkamp J, Westenberg JJ, Bax JJ, Kroft LJ, de Roos A. Reduced aortic elasticity and dilatation are associated with aortic regurgitation and left ventricular hypertrophy in nonstenotic bicuspid aortic valve patients. J Am Coll Cardiol 2007;49:1660-5. [Crossref] [PubMed]
- Gibbons RJ, Valeti US, Araoz PA, Jaffe AS. The quantification of infarct size. J Am Coll Cardiol 2004;44:1533-42. [Crossref] [PubMed]
- Fairbairn TA, Steadman CD, Mather AN, Motwani M, Blackman DJ, Plein S, McCann GP, Greenwood JP. Assessment of valve haemodynamics, reverse ventricular remodelling and myocardial fibrosis following transcatheter aortic valve implantation compared to surgical aortic valve replacement: a cardiovascular magnetic resonance study. Heart 2013;99:1185-91. [Crossref] [PubMed]
- Kim WK, Rolf A, Liebetrau C, Van Linden A, Blumenstein J, Kempfert J, Bachmann G, Nef H, Hamm C, Walther T. M?llmann H. Detection of myocardial injury by CMR after transcatheter aortic valve replacement. J Am Coll Cardiol 2014;64:349-57. [Crossref] [PubMed]
- Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005;26:1461-74. [Crossref] [PubMed]
- Sanz J, Dangas G. Myocardial damage after TAVR assessed with CMR: a new piece in a puzzle? J Am Coll Cardiol 2014;64:358-60. [Crossref] [PubMed]
- La Manna A, Sanfilippo A, Capodanno D, Salemi A, Cadoni A, Cascone I, Polizzi G, Figuera M, Pittalà R, Privitera C, Tamburino C. Left ventricular reverse remodeling after transcatheter aortic valve implantation: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2013;15:39. [Crossref] [PubMed]
- Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation 2013;127:397-407. [Crossref] [PubMed]
- Stähli BE, Maier W, Corti R, Lüscher TF, Jenni R, Tanner FC. Aortic regurgitation after transcatheter aortic valve implantation: mechanisms and implications. Cardiovasc Diagn Ther 2013;3:15-22. [PubMed]
- Athappan G, Patvardhan E, Tuzcu EM, Svensson LG, Lemos PA, Fraccaro C, Tarantini G, Sinning JM, Nickenig G, Capodanno D, Tamburino C, Latib A, Colombo A, Kapadia SR. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. [Crossref] [PubMed]
- Kodali S, Pibarot P, Douglas PS, Williams M, Xu K, Thourani V, Rihal CS, Zajarias A, Doshi D, Davidson M, Tuzcu EM, Stewart W, Weissman NJ, Svensson L, Greason K, Maniar H, Mack M, Anwaruddin S, Leon MB, Hahn RT. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J 2015;36:449-56. [Crossref] [PubMed]
- Crouch G, Tully PJ, Bennetts J, Sinhal A, Bradbrook C, Penhall AL, De Pasquale CG, Baker RA, Selvanayagam JB. Quantitative assessment of paravalvular regurgitation following transcatheter aortic valve replacement. J Cardiovasc Magn Reson 2015;17:32. [Crossref] [PubMed]
- Pibarot P, Hahn RT, Weissman NJ, Monaghan MJ. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging 2015;8:340-60. [Crossref] [PubMed]
- Altiok E, Frick M, Meyer CG, Al Ateah G, Napp A, Kirschfink A, Almalla M, Lotfi S, Becker M, Herich L, Lehmacher W, Hoffmann R. Comparison of two- and three-dimensional transthoracic echocardiography to cardiac magnetic resonance imaging for assessment of paravalvular regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2014;113:1859-66. [Crossref] [PubMed]
- Singh A, Steadman CD, Khan JN, Horsfield MA, Bekele S, Nazir SA, Kanagala P, Masca NG, Clarysse P, McCann GP. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 2015;41:1129-37. [Crossref] [PubMed]
- Swoboda PP, Larghat A, Zaman A, Fairbairn TA, Motwani M, Greenwood JP, Plein S. Reproducibility of myocardial strain and left ventricular twist measured using complementary spatial modulation of magnetization. J Magn Reson Imaging 2014;39:887-94. [Crossref] [PubMed]
- Sandstede JJ, Johnson T, Harre K, Beer M, Hofmann S, Pabst T, Kenn W, Voelker W, Neubauer S, Hahn D. Cardiac systolic rotation and contraction before and after valve replacement for aortic stenosis: a myocardial tagging study using MR imaging. AJR Am J Roentgenol 2002;178:953-8. [Crossref] [PubMed]
- Biederman RW, Doyle M, Yamrozik J, Williams RB, Rathi VK, Vido D, Caruppannan K, Osman N, Bress V, Rayarao G, Biederman CM, Mankad S, Magovern JA, Reichek N. Physiologic compensation is supranormal in compensated aortic stenosis: does it return to normal after aortic valve replacement or is it blunted by coexistent coronary artery disease? An intramyocardial magnetic resonance imaging study. Circulation 2005;112:I429-36. [PubMed]
- Meyer CG, Frick M, Lotfi S, Altiok E, Koos R, Kirschfink A, Lehrke M, Autschbach R, Hoffmann R. Regional left ventricular function after transapical vs. transfemoral transcatheter aortic valve implantation analysed by cardiac magnetic resonance feature tracking. Eur Heart J Cardiovasc Imaging 2014;15:1168-76. [Crossref] [PubMed]
- McGavock JM, Victor RG, Unger RH, Szczepaniak LS; American College of Physicians and the American Physiological Society. Adiposity of the heart, revisited. Ann Intern Med 2006;144:517-24. [Crossref] [PubMed]
- Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, Siebelink HM, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010;122:2538-44. [Crossref] [PubMed]
- Mahmod M, Bull S, Suttie JJ, Pal N, Holloway C, Dass S, Myerson SG, Schneider JE, De Silva R, Petrou M, Sayeed R, Westaby S, Clelland C, Francis JM, Ashrafian H, Karamitsos TD, Neubauer S. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ Cardiovasc Imaging 2013;6:808-16. [Crossref] [PubMed]
- Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab 2012;15:805-12. [Crossref] [PubMed]
- Stankovic Z, Allen BD, Garcia J, Jarvis KB, Markl M. 4D flow imaging with MRI. Cardiovasc Diagn Ther 2014;4:173-92. [PubMed]
- Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll CJ, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, Hope MD, Kilner PJ, Kozerke S, Myerson S, Neubauer S, Wieben O, Markl M. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015;17:72. [Crossref] [PubMed]
- Markl M, Chan FP, Alley MT, Wedding KL, Draney MT, Elkins CJ, Parker DW, Wicker R, Taylor CA, Herfkens RJ, Pelc NJ. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging 2003;17:499-506. [Crossref] [PubMed]
- Markl M, Wallis W, Harloff A. Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI. J Magn Reson Imaging 2011;33:988-94. [Crossref] [PubMed]
- Bock J, Frydrychowicz A, Lorenz R, Hirtler D, Barker AJ, Johnson KM, Arnold R, Burkhardt H, Hennig J, Markl M. In vivo noninvasive 4D pressure difference mapping in the human aorta: phantom comparison and application in healthy volunteers and patients. Magn Reson Med 2011;66:1079-88. [Crossref] [PubMed]
- Dyverfeldt P, Gårdhagen R, Sigfridsson A, Karlsson M, Ebbers T. On MRI turbulence quantification. Magn Reson Imaging 2009;27:913-22. [Crossref] [PubMed]
- Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, Malaisrie SC, McCarthy P, Collins J, Carr J, Markl M. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014;129:673-82. [Crossref] [PubMed]
- von Knobelsdorff-Brenkenhoff F, Trauzeddel RF, Barker AJ, Gruettner H, Markl M, Schulz-Menger J. Blood flow characteristics in the ascending aorta after aortic valve replacement--a pilot study using 4D-flow MRI. Int J Cardiol 2014;170:426-33. [Crossref] [PubMed]
- Trauzeddel RF, Löbe U, Barker AJ, Gelsinger C, Butter C, Markl M, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F. Blood flow characteristics in the ascending aorta after TAVI compared to surgical aortic valve replacement. Int J Cardiovasc Imaging 2016;32:461-7. [Crossref] [PubMed]


