
LI-RADS LR-5 on contrast-enhanced ultrasonography has satisfactory diagnostic specificity for hepatocellular carcinoma: a systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is acknowledged for its insidious onset, rapid progression, high rate of post-surgical recurrence, and frequent intrahepatic and extrahepatic metastases. Globally, HCC ranks sixth among all cancers for disease morbidity and third for tumor-related mortality (1). Owing to the characteristic imaging patterns of HCC, most patients are diagnosed before surgery on the basis of radiologic findings detected during enhanced computed tomography (CT) or enhanced magnetic resonance imaging (MRI) examinations, for example (2,3). Moreover, the tendency of uncontrollable hemorrhage, the possibility of biopsy-related tumor metastasis, and the likelihood of false negative results due to the size and location of nodules mean that imaging techniques are safer and more convenient pre-surgical procedures for the diagnosis and evaluation of HCC than other diagnostic methods such as needle biopsy (4).
Despite the advancement in liver imaging techniques and increasing levels of expertise, the identification and assessment of small liver nodules—especially those of less than 2 cm in diameter—remains a challenge (5). Misdiagnosis or false negative diagnosis of suspected nodules impedes early radical surgery and greatly contributes to the high mortality of HCC. Further, incorrect classification of benign lesions, such as hepatitis B virus-related nodules and hepatic hemangioma, as malignant causes patients pain and suffering, and has occurred persistently in clinical practice (6).
Besides enhanced CT and MRI, contrast-enhanced ultrasonography (CEUS) has increasingly been applied in both the screening of populations at high risk of HCC and the assessment of suspected liver nodules, mainly due to its potential to enable the dynamic observation of a particular liver nodule during the influx, dispersion, and absorption of the contrast agent (7). With the purpose of boosting the accuracy of diagnosis by raising the sensitivity and specificity, and promoting a more professional and optimized diagnostic process, the Liver Imaging Reporting and Data System (LI-RADS) for CEUS was introduced in 2016 by the American College of Radiology (ACR). Using the contrast agent SonoVue (Bracco Imaging S.p.A., Milan, Italy), this system categorizes liver nodules based on size, pattern of enhancement in the arterial phase, type of washout, as well as the duration between enhancement and washout (8). The system categorizes nodules into five categories which reflect the relative probability of HCC (LR-1 to 5) along with the category of LR-M (probable malignancy but not HCC-specific). In 2017, another updated version of the LI-RADS by CEUS was published to improve the diagnostic efficiency (9).
Determining the substance of liver nodules is of paramount significance to evaluating the necessity of radical surgery. According to the LI-RADS, the LR-5 category on CEUS is highly indicative of HCC and may be a sufficient indication for surgical removal, providing that the mass is considered resectable on the basis of its size, location, relationship to other tissues, and possibility of remote metastasis, etc. (10,11). Therefore, the accurate, precise, and timely recognition of LR-5 nodules is mandatory for promoting early resection and a preferable prognosis. To date, several studies have examined the diagnostic efficiency of LR-5 for HCC (12-23). However, these studies have provided relatively inconsistent results for diagnostic sensitivity and specificity. Moreover, the characteristics of patients have also been significantly inconsistent between studies. For example, over one-half of the patients included in Terzi et al.’s study had a background of hepatitis C virus (HCV)-related liver cirrhosis, which represented a larger proportion of the study population than in other studies (22).
While two high-quality meta-analyses have been carried out to determine the exact diagnostic performance of enhanced MRI LI-RADS, similar comprehensive studies regarding CEUS LI-RADS are scarce (24,25). As mentioned above, the determination of LR-5 nodules is a crucial step in a patient’s long-term treatment and rehabilitation; therefore, it is necessary to undertake further research to collect evidence of the exact diagnostic efficiency of the CEUS LI-RADS LR-5 category in terms of sensitivity and specificity (26). To this end, we conducted a systematic review and meta-analysis based on already published data to determine the diagnostic efficiency of the CEUS LR-5 category for HCC. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-591/rc).
Methods
We registered this study on the International Platform of Registered Systematic Review and Meta-analysis Protocols (registration No. INPLASY2022100011).
Search of the literature
To avoid missing important studies for inclusion in the literature review, we thoroughly searched for relevant clinical research articles reporting on the diagnostic efficiency of CEUS LR-5 for HCC in major medical databases including PubMed, Ovid, Medline, Embase, the Cochrane Library, and Web of Science. For thoroughness, we also performed a literature search in some of the Chinese medical databases, such as the CNKI (China National Knowledge Infrastructure) and Wanfang Data. The English language search words were (“hepatocellular carcinoma” OR “liver cancer” OR “liver tumor” OR “liver nodule” OR “liver mass” OR “liver lesion” OR “HCC”) AND (“contrast-enhanced US” OR “contrast-enhanced ultrasonography” OR “contrast-enhanced ultrasound” OR “CEUS”) AND (“LI-RADS” OR “liver reporting and data system”). Since LI-RADS was first introduced in 2016 and refined in 2017, we limited the publication time from January 2017 to June 2021. Studies written in languages other than English were not considered for inclusion in this meta-analysis.
Eligibility criteria
After a primary search for studies in the selected databases, we removed articles which were deemed unnecessary or irrelevant to the meta-analysis. This process was carefully carried out following strict inclusion and exclusion criteria by HJ and MZ under the supervision of LH. The inclusion criteria for articles were as follows: (I) contained data directly relating to the diagnostic efficiency of LR-5 for HCC in patients with undefined but suspected liver nodules; (II) the full text could be downloaded and appraised; and (III) pathological examination had been used as the gold standard for assessing the diagnostic efficacy of CEUS LR-5 for HCC.
The main exclusion criteria included the following: (I) the standard version of LI-RADS was not applied in the imaging diagnosis; (II) an inadequate number of lesions (<50) was reported; and (III) the exact numbers of positive or negative diagnoses by LR-5 and by pathological examination were not reported, meaning the sensitivity and specificity could not be pooled.
Extraction of data
After the completion of the article selection process, the full texts were acquired and scrupulously read. Data on the outcome indicators of this meta-analysis, including true negative (TN), false negative (FN), true positive (TP), false positive (FP), and diagnostic accuracy values, were primarily extracted. Other fundamental information about each study was also extracted, including the country where the study was conducted, first author, year of publication, study type, study design, number of medical centers involved, number of patients enrolled (including year of enrollment, mean age, and sex), number of liver lesions, average lesion size, number of ultrasound systems, type of contrast agent, version of CEUS LI-RADS, and reference standard. Two reviewers were appointed to extract the data (YC and QH), and a third reviewer (WB) helped to settle any disagreements that occurred.
Quality assessment
To ensure the quality of the pooled results in this study, particular attention was paid to the quality of the studies selected for meta-analysis. Studies with potential problems concerning risk of bias were no longer considered. In this process, we mainly referred to the QUADAS-2 evaluation system (27). This work was undertaken collaboratively by LZ and XC under the guidance of WL.
Statistical analysis
Since some studies reported on imaging classification performed by two or more radiology experts, we treated data from a particular expert as an individual unit when pooling the results. The meta-analysis was performed using Meta-DiSc 1.4 (Ramóny Cajal Hospital, Madrid, Spain). In this process, the hierarchical summary receiver operating characteristic (HSROC) model and the area under curve were applied and calculated to help determine the diagnostic efficacy of CEUS LR-5 for HCC. A meta-regression analysis was also conducted to identify factors that potentially contributed to the heterogeneity between studies.
Results
Literature selection
A total of 226 records were identified in the international and Chinese medical databases. After the removal of 83 duplicated records, 143 studies remained. A further 78 studies were removed following an assessment of study relevancy. Subsequently, 8 studies were excluded due to the unavailability of their full texts, and 28 studies were excluded due to being case reports, letters, editorials, comments, animal studies, or reviews. After the exclusion of 5 articles without adequate lesions and 3 studies with overlapping data, 12 studies were finally deemed eligible for inclusion in the meta-analysis. The steps of article screening are shown in Figure 1.
Study characteristics
Twelve eligible studies with full-texts and original TP, TN, FP, and FN data were incorporated into the meta-analysis (12-23). Among the studies, 7 were carried out in China (12-14,16,17,19,20), 1 in Canada (15), 2 in Germany (21,23), 1 in Italy (22), and 1 in Korea (18). Two of the studies were prospective (18, 23), while the other 10 were retrospective (12-17,19-22). Two of the studies were launched in multiple centers (17,22) and the remaining 10 studies (12-16,18-21,23) were conducted in single (hospital) centers. Eleven studies (12-17,19-23) used SonoVue (Bracco Imaging S.p.A., Milan, Italy) as the contrast agent, and 1 study used SonoVue and Sonazoid (GE Healthcare, Milwaukee, WI, USA) (18). The reference standards were pathology, composite imaging, and follow-up. The details of the studies that were finally included are recorded in Table 1.
Table 1
| Study | Author/year | Country | Study design | Study type | Center | No. of patients | Patient age | Patient sex (male/female) | Years of enrollment | No. of lesions | Lesion size [mm] | No. of HCC lesions | Number of non-HCC malignancies | No. of benign lesions | No. of US systems | Contrast agent | LI-RADS version | Reference standard |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ding 2021 (12) | China | Cohort | Retrospective | Single center | 239 | 59.1 [51.1–67.1] | 182/57 | June 2017–Jan 2019 | 273 | 30.7 [10–61] | 225 | 22 (ICC: 12; cHCC-CCA: 8; metastasis: 2) | 26 | Single system | SonoVue | 2017 | Pathology |
| 2 | Wang 2020 (13) | China | Cohort | Retrospective | Single center | 258 | 52 [21–82] | 192/66 | Mar 2015–Nov 2017 | 355 | 25 [6–183] | 115 | 5 (ICC: 2; cHCC-CCA: 1; others: 2) | 235 | Single system | SonoVue | 2017 | Pathology, imaging and follow-up |
| 3 | Huang 2020 (14) | China | Cohort | Retrospective | Single center | 172 | 52 [21–78] | 136/36 | Jan 2015–Feb 2018 | 175 | 16 [8–20] | 105 | 3 (ICC: 2; others: 1) | 67 | Single system | SonoVue | 2017 | Pathology, imaging and follow-up |
| 4 | Makoyeva 2020 (15) | Canada | Cohort | Retrospective | Single center | 184 | 62 [27–87] | 138/46 | 2008–2016 | 196 | NA | 139 | 18 (ICC: 9; cHCC-CCA: 1; metastasis: 6; others: 2) | 39 | Multiple systems | SonoVue | 2016 | Pathology, imaging and follow-up |
| 5 | Zheng 2020 (16) | China | Cohort | Retrospective | Single center | 1826 | 64 [44–62] | 1642/184 | Jan 2004–Dec 2016 | 2020 | NA | 1514 | 138 (ICC: 57; cHCC-CCA: 24; metastasis: 53; others:4) | 368 | Single system | SonoVue | 2017 | Pathology, imaging and follow-up |
| 6 | Zhou 2022 (17) | China | Cohort | Retrospective | Multi-center | 96 | 58.5 [35–87] | 81/15 | May 2017–Dec 2018 | 96 | 42.7 [9.9–169] | 67 | 22 (NA) | 7 | NA | SonoVue | 2017 | Pathology |
| 7 | Kang 2020 (18) | Korea | Cohort | Prospective | Single center | 59 | 65 [49–86] | 47/12 | Feb 2019–Aug 2019 | 59 | 10–50 mm: 52; >50 mm: 7 | 43 | 10 (ICC: 6: cHCC-CCA: 3; others: 1) | 6 | Multiple systems | SonoVue/Sonozoid | 2017 | Pathology, imaging and follow-up |
| 8 | Li 2019 (19) | China | Cohort | Retrospective | Single center | 1366 | 52 [NA] | 1097/269 | Jan 2014–Dec 2017 | 1366 | 35 [5–200] | 985 | 139 (ICC: 59; cHCC-CCA: 14; Metastasis: 62; Others: 4) | 242 | Single system | SonoVue | 2017 | Pathology |
| 9 | Chen 2019 (20) | China | Case-control | Retrospective | Single center | 210 | 55 [32–84] | 163/47 | Nov 2003–Dec 2017 | 210 | ≤30 mm: 25; 31–50 mm: 47; >50 mm: 138 | 105 | 105 (ICC: 105) | 0 | Three systems | SonoVue | 2017 | Pathology |
| 10 | Schellhaas 2018 (21) | Germany | Cohort | Retrospective | Single center | 39 | 66 [53–86] | NA | NA | 55 | NA | 48 | 2 (ICC: 2) | 5 | Single system | SonoVue | 2016 | Pathology, imaging and follow-up |
| 11 | Terzi 2018 (22) | Italy | Cohort | Retrospective | Multi-center | 848 | 70 [31–89] | 457/391 | Jan 2005–Dec 2015 | 1006 | 20 [5–150] | 820 | 53 (ICC: 40; cHCC-CCA: 9; others: 4) | 133 | Multiple systems | SonoVue | 2016 | Pathology, imaging and follow-up |
| 12 | Schellhaas 2017 (23) | Germany | Cohort | Prospective | Single center | 100 | 66 [42–85] | 85/15 | NA | 100 | 52.2 [10–290] | 87 | 6 (ICC: 6) | 7 | Three systems | SonoVue | 2016 | Pathology, imaging and follow-up |
HCC, hepatocellular carcinoma; US, ultrasound; LI-RADS, liver imaging reporting and data system; NA, not available; ICC, intrahepatic cholangiocarcinoma; cHCC-CCA, combined hepatocellular-cholangiocarcinoma.
Characteristics of patients and lesions
In summary, 5397 patients with 5911 focal liver lesions were taken into consideration in this meta-analysis. The studies conducted by Zheng et al. (16) (1,826 patients and 2,020 lesions) and Li et al. (19) (1,366 patients and 1,366 lesions) included the largest number of patients and lesions. The study with the fewest patients was that of Schellhaas et al. (21) (39 patients and 55 lesions). Patient information, including sex, age, years of study enrollment, and lesion size and pathology, are shown in Table 1.
Quality assessment
All studies underwent quality assessment procedures according to the QUADAS-2 protocol. The 12 included studies were generally found to be high-quality studies, with low risk for patient selection, index test, reference standard, and flow and timing. Only 2 studies had unclear risk for patient selection in terms of risk of bias, and only 2 studies had unclear risk for patient selection and index test in terms of applicability. However, although the 12 studies were of a relatively high quality, there did exist concerns regarding the index test domain. For instance, several of the studies chose pathology as the only standard reference, while some studies reported incorporated results for other imaging techniques and long-term follow-up. Moreover, several studies failed to report the exact interval between the index test and the reference standard. The detailed quality evaluation by QUADAS-2 is shown in Figure S1.
Diagnostic performance of LR-5
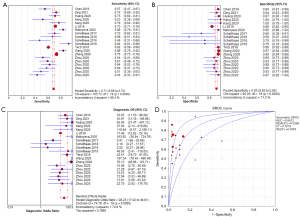
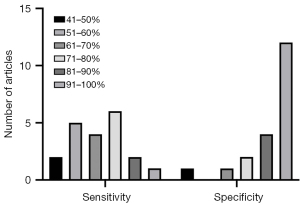
The forest plot of the pooled sensitivity of LR-5 is shown in Figure 2A. The pooled sensitivity from 20 radiologists (Schellhaas et al. reported results from 3 radiologists; Zhou et al. reported results from 6 radiologists) was 0.71 [95% confidence interval (CI): 0.69–0.72] with heterogeneity (I2) of 88.4%. The highest sensitivity recorded was 0.94 (95% CI: 0.81–0.99) by one of the experts in Schellhaas et al.’s study, and the lowest sensitivity was 0.45 (95% CI: 0.33–0.57) from an expert in Zhou et al.’s study. Ten of the 20 sensitivity data items ranged from 61% to 80%. The forest plot of LR-5 specificity is shown in Figure 2B. The pooled specificity from the 20 radiologists was 0.93 (95% CI: 0.92–0.95), with an I2 of 71.2%. The highest specificity recorded was 1.00 (95% CI: 0.79–1.00) by an expert in the Kang et al.’s study, and the lowest specificity recorded was 0.50 (95% CI: 0.07–0.93) by an expert in Schellhaas et al.’s study. In 16 out of 20 cases, the specificity exceeded 81%, which is high. The pooled diagnostic odds ratio (Figure 2C) was 28.23 (95% CI: 17.02–46.81), with an I2 of 74.6%. The pooled positive likelihood ratio was 8.62 (95% CI: 5.55–13.38), with an I2 of 76.8%. The pooled negative likelihood ratio was 0.35 (95% CI: 0.30–0.41), with an I2 of 88.4%. The summary receiver operating characteristic (SROC) curve was drawn, and the area under the curve was 0.8612. The SROC curve is shown in Figure 2D. The distribution of sensitivity and specificity is shown in Figure 3.
Meta-regression analysis
Various covariates were carefully analyzed with respect to their relationship to the pooled study heterogeneity. They included study design (case-control or cohort study), study type (prospective or retrospective), number of medical centers involved, number of patients enrolled, number of lesions, number of ultrasound systems, contrast agents, LI-RADS version, and reference standard. Among the above-mentioned covariates, the number of medical centers (P=0.009) and the reference standard (P=0.035) were statistically correlated with study heterogeneity. Specifically, combined use of pathology and imaging follow-up as the reference standard had a higher sensitivity than did pathology alone (0.73 vs. 0.60, P=0.035). Also, studies conducted in a single medical center were found to have higher sensitivity than those conducted in multiple medical centers (0.72 vs. 0.57, P=0.009). The results of the meta-regression analysis are reported in Table 2.
Table 2
| Covariates | Subgroup | No. of experts reading | Meta-analysis summary estimate | |||
|---|---|---|---|---|---|---|
| Sensitivity | P value | Specificity | P value | |||
| Study design | Cohort | 19 | 0.67 (95% CI: 0.61–0.74) | 0.48 | 0.89 (95% CI: 0.83–0.94) | 0.58 |
| Case control | 1 | 0.57 (95% CI: 0.47–0.67) | 0.96 (95% CI: 0.91–0.99) | |||
| Study type | Retrospective | 17 | 0.67 (95% CI: 0.60–0.73) | 0.88 | 0.89 (95% CI: 0.83–0.95) | 0.92 |
| Prospective | 3 | 0.68 (95% CI: 0.53–0.83) | 0.90 (95% CI: 0.81–0.99) | |||
| Center | Single center | 13 | 0.72 (95% CI: 0.65–0.80) | 0.009 | 0.87 (95% CI: 0.79–0.95) | 0.35 |
| Multi-center | 7 | 0.57 (95% CI: 0.52–0.62) | 0.93 (95% CI: 0.83–0.99) | |||
| No. of patients | <100 | 11 | 0.62 (95% CI: 0.53–0.70) | 0.056 | 0.87 (95% CI: 0.78–0.95) | 0.367 |
| ≥100 | 9 | 0.73 (95% CI: 0.67–0.79) | 0.92 (95% CI: 0.86–0.98) | |||
| No. of lesions | <100 | 11 | 0.62 (95% CI: 0.53–0.70) | 0.056 | 0.87 (95% CI: 0.78–0.95) | 0.367 |
| ≥100 | 9 | 0.73 (95% CI: 0.67–0.79) | 0.92 (95% CI: 0.86–0.98) | |||
| Number of US systems | Single system | 14 | 0.66 (95% CI: 0.59–0.74) | 0.792 | 0.87 (95% CI: 0.81–0.94) | 0.387 |
| Multiple systems | 6 | 0.68 (95% CI: 0.58–0.79) | 0.93 (95% CI: 0.88–0.98) | |||
| Contrast agent | SonoVue | 18 | 0.67 (95% CI: 0.61–0.73) | 0.924 | 0.88 (95% CI: 0.81–0.94) | <0.001 |
| SonoVue & Sonozoid | 2 | 0.66 (95% CI: 0.41–0.91) | 1.00 | |||
| LI-RADS version | v. 2016 | 6 | 0.72 (95% CI: 0.59–0.85) | 0.281 | 0.77 (95% CI: 0.63–0.91) | 0.058 |
| v. 2017 | 14 | 0.65 (95% CI: 0.58–0.71) | 0.94 (95% CI: 0.92–0.96) | |||
| Reference standard | Pathology only | 9 | 0.60 (95% CI: 0.54–0.66) | 0.035 | 0.92 (95% CI: 0.90–0.94) | 0.331 |
| Pathology & imaging & follow-up | 11 | 0.73 (95% CI: 0.64–0.81) | 0.86 (95% CI: 0.77–0.96) | |||
CI, confidence interval; US, ultrasound; LI-RADS, Liver Imaging Reporting and Data System.
Discussion
In this meta-analysis, we included 12 studies (7 of which were conducted after 2020) on the diagnostic efficiency of CEUS LI-RADS LR-5 for HCC, as measured by sensitivity and specificity. Through our analysis, we found that CEUS LI-RADS LR-5 had a suboptimal pooled diagnostic sensitivity of 0.71 of LR-5 for HCC but a high pooled diagnostic specificity of 0.93, which proved that LR-5 can reduce the false positive rate to a large extent. This result indicates that the present approach to directly diagnosing HCC on the basis of the LI-RADS LR-5 category observed on CEUS, without pathological evidence, is quite reliable. However, we acknowledge that the existence of some heterogeneity between the studies in the meta-analysis might have influenced the outcome.
According to past research concerning the diagnostic performance of CT/MRI LI-RADS, the diagnostic sensitivity and specificity were higher in our study (71% and 93% respectively) than in the previous meta-analysis of CT/MRI LI-RADS (62% and 92%, respectively), which suggests that the diagnostic efficiency of CEUS is comparable to that of CT/MRI (28). Furthermore, the fact that CEUS is safer, cheaper, and more convenient supports its value in both the early diagnosis and final diagnosis of HCC (14,18,25-26). However, researchers have pointed out that CEUS also has some shortcomings. First and foremost, observation of the liver through CEUS may be affected by air in the thorax, resulting in poor visualization of masses near the diaphragm (29). Furthermore, masses located deep in the liver (>12 cm), especially those in the right lobe, are sometimes missed on CEUS (30). Finally, observations on CEUS can be affected by a patient’s failure to inhale and exhale in accordance with instruction, especially when the patient is older and has concomitant cardio-pulmonary insufficiency (31).
However, despite the above shortcomings, CEUS did show higher sensitivity and specificity in our meta-analysis than it did in other meta-analyses regarding CT/MRI LI-RADS (28). Therefore, due to the specific principles of LI-RADS of CEUS and CT/MRI, CEUS might supplement the clinical value than CT/MRI, displaying a lower missed diagnosis rate and a lower false positive rate (32). Furthermore, according to the still and pre-set imaging techniques in a previous study, CT/MRI was not able to observe the existence of hyperenhancement in both the early and late arterial phase, which was possible in the CEUS examination (33). Consequently, the CEUS LI-RADS might increase diagnostic accuracy by providing more detailed and specific judging criteria than the CT/MRI LI-RADS. Also, according to the 2017 version of the CEUS LI-RADS, the LR-5 category is assigned when lesions are bigger than 10 mm and show arterial-phase hyperenhancement with washout, while the LR-4 category is assigned based on the same conditions but in the absence of washout (34). However, the 2018 version of the CT/MRI LI-RADS assigns LR-5 based on the comparatively rigorous criteria of lesion size between 10 and 20 mm, with non-rim arterial-phase hyperenhancement, and non-peripheral washout (35). Therefore, this difference might have contributed to the comparatively high sensitivity of CEUS LI-RADS LR-5 for HCC observed in this study. From another perspective, the specific physical, chemical, and biological features of contrast agents can result in potentially different patterns in imaging signs. The contrast agents used for CEUS manifest as intravascular microbubbles, which show clearer and more typical enhancement patterns of HCC and intrahepatic cholangiocarcinoma, and thus, may contribute to higher diagnostic accuracy and specificity (36,37). Moreover, since CEUS and CT/MRI rely on different diagnostic parameters due to distinct image acquisition techniques and the properties of contrast agents, a couple of studies have suggested that CEUS should be supplemented if results of more than 2 imaging techniques contradicted (38).
As reported before, diagnostic sensitivity, specificity, and accuracy may be influenced by several factors, such as features of lesions and concomitant underlying chronic liver diseases (39). Important features of lesions, including location, size, and differentiation stage, result in visual differences (40). Specifically, lesions close to the diaphragm (observation of which can be affected by air in the thorax), lesions in the lateral portion of the left liver and in the lower, lateral portion of the right liver (observation of which can be affected by air in the gastrointestinal tract), lesions that are small in size (less than 1.5 cm), and lesions with a high differentiation status can result in high false negative rates (41). Also, one article included in this meta-analysis reported the use of Sonazoid (as perfluorobutane microbubbles) as the contrast agent in CEUS, with two independent experts recording sensitivities of 0.53 and 0.79, respectively, and specificities of 1.00 and 1.00, respectively, which represents satisfactory sensitivity and extremely high specificity.
To date, a dozen studies have been implemented to measure the relative diagnostic efficacy of the two commonly applied contrast agents in CEUS, SonoVue (Bracco Imaging S.p.A., Milan, Italy) and Sonazoid (GE Healthcare, Milwaukee, WI, USA) (42,43). In one study, SonoVue typically provided a vascular phase and post-vascular phase in which early hyperenhancement and fast washout could be observed in HCC nodules (44). However, atypical HCC lesions with late washout during the delayed phase could profoundly contribute to the augmentation of false negative cases. Like CEUS with SonoVue, CEUS with Sonazoid enables vascular phase imaging; however, it has the additional advantage of allowing for Kupffer phase imaging, which typically starts from 10 mins to 15 mins after Sonazoid injection. In the normal liver parenchyma, Kupffer cells absorb Sonazoid so that the liver shows enhancement during the Kupffer phase (45). However, HCC lesions which do not contain Kupffer cells can manifest as defects in the Kupffer phase. Perfusion defects in the Kupffer phase greatly facilitate and promote the characterization of HCC, even in lesions with atypical arterial phase and delayed phase manifestations. In recent years, an increasing number of studies have encouragingly discovered comparable diagnostic accuracy between CEUS with Sonazoid and with Sonovue, which has provided physicians with more options in the selection of contrast agents. In 2018, Zhai et al. recruited 65 patients with HCC to compare the function of CEUS using Sonozoid and SonoVue. Their results demonstrated that Sonozoid and Sonovue increased the diagnostic accuracy for HCC nodules by 30% and 16%, respectively (46). In another prospective study conducted in 17 medical centers in China and Korea, three experienced ultrasound readers reported increases in diagnostic accuracy of 24.2%, 14.6%, and 16.7% for SonoVue, and 18.1%, 22.1%, and 17.0% for Sonazoid, compared with B-mode ultrasound (47). In 2020, a systematic review included three studies with outcomes of diagnostic accuracy for CEUS with Sonazoid and one study of CEUS with SonoVue (48). The diagnostic accuracies of Sonazoid reported in the three studies were 92% (65 lesions), 95% (41 lesions), and 88% (16 lesions), and the diagnostic accuracy of SonoVue was 98% (86 lesions). Therefore, we believe that Sonazoid is a strong supplemental contrast agent for CEUS in the diagnosis of HCC, especially in the case of atypical nodules which are either difficult to distinguish under B-mode ultrasound or which do not manifest with fast washout or early arterial hyperenhancement in the pure vascular phase of SonoVue. More importantly, Sonazoid is also preferable because it offers the potential to conduct repeated and multiple scans during the Kupffer phase, owing to its tolerability and stability. Specifically, research has shown that a supplementary injection of Sonazoid can be given to promote reperfusion in the defect area during the Kupffer phase and, if early-stage wash-in reappears in the defect area during the new vascular stage, HCC can be diagnosed (49).
Since the studies in our meta-analysis used distinct study designs and methods, between-study heterogeneity was inevitable. By meta-regression analysis, both the number of centers and the reference standard were found to have directly led to the study heterogeneity observed. In this meta-regression analysis, 9 observations were compared to pathological results only, while the other 11 observations were compared to results of pathology combined with imaging examination and long-term follow-up. The 11 observations which were compared to both pathological and imaging results yielded much higher sensitivity than did the 9 observations that were compared to pathological results alone. Since CEUS might have been used as a technique in imaging examination, we therefore supposed that reference standard bias might exist.
To the best of our knowledge, our study incorporated recent studies published after June 2020, and thus provides more accurate and comprehensive information on the topic. Still, we acknowledge several limitations of our study which might have led to bias. Firstly, heterogeneity persisted among the studies, including inconsistency in patient numbers, patient ages, and tumor sizes. Secondly, the number of clinical trials published within the date range was small, which might amplify the effects of individual studies.
Conclusions
The CEUS LI-RADS LR-5 category demonstrated a satisfactory diagnostic specificity for HCC but had suboptimal diagnostic sensitivity. Study heterogeneity was observed and was found to be related to the number of medical centers involved as well as the reference standard.
Acknowledgments
Funding: This study received funding by the Miaozi Project in Science and Technology Innovation Program of Sichuan Province (No. 2020079) and Sichuan Science and Technology Program (No. 2020YFS0211).
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-591/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-591/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021;73:4-13. [Crossref] [PubMed]
- Liava C, Sinakos E, Papadopoulou E, Giannakopoulou L, Potsi S, Moumtzouoglou A, Chatziioannou A, Stergioulas L, Kalogeropoulou L, Dedes I, Akriviadis E, Chourmouzi D. Liver Imaging Reporting and Data System criteria for the diagnosis of hepatocellular carcinoma in clinical practice: A pictorial minireview. World J Gastroenterol 2022;28:4540-56. [Crossref] [PubMed]
- Chartampilas E, Rafailidis V, Georgopoulou V, Kalarakis G, Hatzidakis A, Prassopoulos P. Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers (Basel) 2022.
- Renzulli M, Pecorelli A, Brandi N, Brocchi S, Tovoli F, Granito A, Carrafiello G, Ierardi AM, Golfieri R. The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? J Clin Med 2022; [Crossref] [PubMed]
- Deng H, Shi H, Lei J, Hu Y, Li G, Wang C. A meta-analysis of contrast-enhanced ultrasound for small hepatocellular carcinoma diagnosis. J Cancer Res Ther 2016;12:C274-6. [Crossref] [PubMed]
- Nadarevic T, Colli A, Giljaca V, Fraquelli M, Casazza G, Manzotti C, Štimac D, Miletic D. Magnetic resonance imaging for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev 2022;5:CD014798. [PubMed]
- Li J, Yang L, Ma L, Lu Q, Luo Y. Diagnostic Accuracy of Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for Differentiating Between Hepatocellular Carcinoma and Other Hepatic Malignancies in High-Risk Patients: A Meta-Analysis. Ultraschall Med 2021;42:187-93. [Crossref] [PubMed]
- American College of Radiology. Contrast-Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) version 2016. Accessed April 03, 2020. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS-v2016
- American College of Radiology. Contrast-Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS) version 2017. Accessed April 03, 2020. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS-v2017
- Giorgio A, De Luca M, Gatti P, Matteucci P, Giorgio V. CEUS LI-RADS Categories to Distinguish Hepatocellular Carcinoma and Non-Hepatocellular Carcinoma Malignancies. Radiology 2020;296:E121-2. [Crossref] [PubMed]
- Terzi E, Giamperoli A, Iavarone M, Leoni S, De Bonis L, Granito A, Forgione A, Tovoli F, Piscaglia F. Prognosis of Single Early-Stage Hepatocellular Carcinoma (HCC) with CEUS Inconclusive Imaging (LI-RADS LR-3 and LR-4) Is No Better than Typical HCC (LR-5). Cancers (Basel) 2022; [Crossref] [PubMed]
- Ding J, Long L, Zhang X, Chen C, Zhou H, Zhou Y, Wang Y, Jing X, Ye Z, Wang F. Contrast-enhanced ultrasound LI-RADS 2017: comparison with CT/MRI LI-RADS. Eur Radiol 2021;31:847-54. [Crossref] [PubMed]
- Wang JY, Feng SY, Xu JW, Li J, Chu L, Cui XW, Dietrich CF. Usefulness of the Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Diagnosing Focal Liver Lesions by Inexperienced Radiologists. J Ultrasound Med 2020;39:1537-46. [Crossref] [PubMed]
- Huang JY, Li JW, Lu Q, Luo Y, Lin L, Shi YJ, Li T, Liu JB, Lyshchik A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology 2020;294:329-39. [Crossref] [PubMed]
- Makoyeva A, Kim TK, Jang HJ, Medellin A, Wilson SR. Use of CEUS LI-RADS for the Accurate Diagnosis of Nodules in Patients at Risk for Hepatocellular Carcinoma: A Validation Study. Radiol Imaging Cancer 2020;2:e190014. [Crossref] [PubMed]
- Zheng W, Li Q, Zou XB, Wang JW, Han F, Li F, Huang LS, Li AH, Zhou JH. Evaluation of Contrast-enhanced US LI-RADS version 2017: Application on 2020 Liver Nodules in Patients with Hepatitis B Infection. Radiology 2020;294:299-307. [Crossref] [PubMed]
- Zhou H, Zhang C, Du L, Jiang J, Zhao Q, Sun J, Li Q, Wan M, Wang X, Hou X, Wen Q, Liu Y, Zhou X, Huang P. Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System in Diagnosing Hepatocellular Carcinoma: Diagnostic Performance and Interobserver Agreement. Ultraschall Med 2022;43:64-71. [Crossref] [PubMed]
- Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrast-enhanced US with Sulfur Hexafluoride and Perfluorobutane for the Diagnosis of Hepatocellular Carcinoma in Individuals with High Risk. Radiology 2020;297:108-16. [Crossref] [PubMed]
- Li J, Ling W, Chen S, Ma L, Yang L, Lu Q, Luo Y. The interreader agreement and validation of contrast-enhanced ultrasound liver imaging reporting and data system. Eur J Radiol 2019;120:108685. [Crossref] [PubMed]
- Chen LD, Ruan SM, Lin Y, Liang JY, Shen SL, Hu HT, Huang Y, Li W, Wang Z, Xie XY, Lu MD, Kuang M, Wang W. Comparison between M-score and LR-M in the reporting system of contrast-enhanced ultrasound LI-RADS. Eur Radiol 2019;29:4249-57. [Crossref] [PubMed]
- Schellhaas B, Pfeifer L, Kielisch C, Goertz RS, Neurath MF, Strobel D. Interobserver Agreement for Contrast-Enhanced Ultrasound (CEUS)-Based Standardized Algorithms for the Diagnosis of Hepatocellular Carcinoma in High-Risk Patients. Ultraschall Med 2018;39:667-74. [Crossref] [PubMed]
- Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 2018;68:485-92. [Crossref] [PubMed]
- Schellhaas B, Görtz RS, Pfeifer L, Kielisch C, Neurath MF, Strobel D. Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: ESCULAP versus CEUS-LI-RADS. Eur J Gastroenterol Hepatol 2017;29:1036-44. [Crossref] [PubMed]
- Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, Ehman EC, Fowler KJ, Hussain HK, Jha RC, Karam AR, Mamidipalli A, Marks RM, Mitchell DG, Morgan TA, Ohliger MA, Shah A, Vu KN, Sirlin CB. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018;286:29-48. [Crossref] [PubMed]
- Kim YY, Lee S, Shin J, Son WJ, Shin H, Lee JE, Hwang JA, Chung YE, Choi JY, Park MS. Diagnostic Performance of Liver Imaging Reporting and Data System Version 2017 Versus Version 2018 for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Comparative Studies. J Magn Reson Imaging 2021;54:1912-9. [Crossref] [PubMed]
- Lee TH, Hirshman N, Cardona DM, Berg CL, Fowler KJ, Bashir MR, Ronald J. LR-3 and LR-4 Lesions Are More Likely to Be Hepatocellular Carcinoma in Transplant Patients with LR-5 or LR-TR Lesions. Dig Dis Sci 2022;67:5345-52. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Lee S, Kim SS, Roh YH, Choi JY, Park MS, Kim MJ. Diagnostic Performance of CT/MRI Liver Imaging Reporting and Data System v2017 for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Int 2020;40:1488-97. [Crossref] [PubMed]
- Caraiani C, Boca B, Bura V, Sparchez Z, Dong Y, Dietrich C. CT/MRI LI-RADS v2018 vs. CEUS LI-RADS v2017-Can Things Be Put Together? Biology (Basel) 2021;10:412. [Crossref] [PubMed]
- Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, Dietrich CF, Kim TK, Willmann JK, Kono Y. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43:127-42. [Crossref] [PubMed]
- Piscaglia F, Wilson SR, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Salvatore V, Willmann JK, Sirlin CB, Kono Y. American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med 2017;38:320-4. [Crossref] [PubMed]
- Shin J, Lee S, Bae H, Chung YE, Choi JY, Huh YM, Park MS. Contrast-enhanced ultrasound liver imaging reporting and data system for diagnosing hepatocellular carcinoma: A meta-analysis. Liver Int 2020;40:2345-52. [Crossref] [PubMed]
- Wang P, Nie F, Dong T, Yang D, Liu T, Wang G. Diagnostic Value of CEUS LI-RADS Version 2017 in Differentiating AFP-Negative Hepatocellular Carcinoma from Other Primary Malignancies of the Liver. Diagnostics (Basel) 2021;11:2250. [Crossref] [PubMed]
- Ciocalteu A, Iordache S, Cazacu SM, Urhut CM, Sandulescu SM, Ciurea AM, Saftoiu A, Sandulescu LD. Role of Contrast-Enhanced Ultrasonography in Hepatocellular Carcinoma by Using LI-RADS and Ancillary Features: A Single Tertiary Centre Experience. Diagnostics (Basel) 2021.
- Patella F, Pesapane F, Fumarola EM, Emili I, Spairani R, Angileri SA, Tresoldi S, Franceschelli G, Carrafiello G. CT-MRI LI-RADS v2017: A Comprehensive Guide for Beginners. J Clin Transl Hepatol 2018;6:222-36. [Crossref] [PubMed]
- Zuo D, Yang K, Wu S. Diagnostic performance of intravascular perfusion based contrast-enhanced ultrasound LI-RADS in the evaluation of hepatocellular carcinoma. Clin Hemorheol Microcirc 2021;78:429-37. [Crossref] [PubMed]
- Huf S, Platz Batista da Silva N, Wiesinger I, Hornung M, Scherer MN, Lang S, Stroszczynski C, Fischer T, Jung EM. Analysis of Liver Tumors Using Preoperative and Intraoperative Contrast-Enhanced Ultrasound (CEUS/IOCEUS) by Radiologists in Comparison to Magnetic Resonance Imaging and Histopathology. Rofo 2017;189:431-40. [Crossref] [PubMed]
- Strobel D, Jung EM, Ziesch M, Praktiknjo M, Link A, Dietrich CF, Klinger C, Schultheiß M, Jesper D, Schellhaas B. Real-life assessment of standardized contrast-enhanced ultrasound (CEUS) and CEUS algorithms (CEUS LI-RADS®/ESCULAP) in hepatic nodules in cirrhotic patients-a prospective multicenter study. Eur Radiol 2021;31:7614-25. [Crossref] [PubMed]
- Sawatzki M, Meyenberger C, Brand S, Semela D. Contrast-enhanced ultrasound (CEUS) has excellent diagnostic accuracy in differentiating focal liver lesions: results from a Swiss tertiary gastroenterological centre. Swiss Med Wkly 2019;149:w20087. [Crossref] [PubMed]
- Pan JM, Chen W, Zheng YL, Cheng MQ, Zeng D, Huang H, Huang Y, Xie XY, Lu MD, Kuang M, Hu HT, Chen LD, Wang W. Tumor size-based validation of contrast-enhanced ultrasound liver imaging reporting and data system (CEUS LI-RADS) 2017 for hepatocellular carcinoma characterizing. Br J Radiol 2021;94:20201359. [Crossref] [PubMed]
- Beyer LP, Wassermann F, Pregler B, Michalik K, Rennert J, Wiesinger I, Stroszczynski C, Wiggermann P, Jung EM. Characterization of Focal Liver Lesions using CEUS and MRI with Liver-Specific Contrast Media: Experience of a Single Radiologic Center. Ultraschall Med 2017;38:619-25. [Crossref] [PubMed]
- Wang F, Numata K, Okada M, Chuma M, Nihonmatsu H, Moriya S, Nozaki A, Ogushi K, Luo W, Ruan L, Nakano M, Otani M, Inayama Y, Maeda S. Comparison of Sonazoid contrast-enhanced ultrasound and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid MRI for the histological diagnosis of hepatocellular carcinoma. Quant Imaging Med Surg 2021;11:2521-40. [Crossref] [PubMed]
- Kang HJ, Lee JM, Yoon JH, Yoo J, Choi Y, Joo I, Han JK. Sonazoid™ versus SonoVue(®) for Diagnosing Hepatocellular Carcinoma Using Contrast-Enhanced Ultrasound in At-Risk Individuals: A Prospective, Single-Center, Intraindividual, Noninferiority Study. Korean J Radiol 2022;23:1067-77. [Crossref] [PubMed]
- Liu L, Tang C, Li L, Chen P, Tan Y, Hu X, Chen K, Shang Y, Liu D, Liu H, Liu H, Nie F, Tian J, Zhao M, He W, Guo Y. Deep learning radiomics for focal liver lesions diagnosis on long-range contrast-enhanced ultrasound and clinical factors. Quant Imaging Med Surg 2022;12:3213-26. [Crossref] [PubMed]
- Li C, Xu J, Liu Y, Wu M, Dai W, Song J, Wang H. Kupffer Phase Radiomics Signature in Sonazoid-Enhanced Ultrasound is an Independent and Effective Predictor of the Pathologic Grade of Hepatocellular Carcinoma. J Oncol 2022;2022:6123242. [Crossref] [PubMed]
- Zhai HY, Liang P, Yu J, Cao F, Kuang M, Liu FY, Liu FY, Zhu XY. Comparison of Sonazoid and SonoVue in the Diagnosis of Focal Liver Lesions: A Preliminary Study. J Ultrasound Med 2019;38:2417-25. [Crossref] [PubMed]
- Lv K, Zhai H, Jiang Y, Liang P, Xu HX, Du L, Chou YH, Xie X, Luo Y, Lee YJ, Lee JY, Hu B, Luo B, Wang Y, Luan Y, Kalli C, Chen K, Wang W, Liang JD. Prospective assessment of diagnostic efficacy and safety of Sonazoid(TM) and SonoVue(®) ultrasound contrast agents in patients with focal liver lesions. Abdom Radiol (NY) 2021;46:4647-59. [Crossref] [PubMed]
- Barr RG, Huang P, Luo Y, Xie X, Zheng R, Yan K, Jing X, Luo Y, Xu H, Fei X, Lee JM. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical evidence for SonoVue and Sonazoid. Abdom Radiol (NY) 2020;45:3779-88. [Crossref] [PubMed]
- Kudo M. Defect Reperfusion Imaging with Sonazoid®: A Breakthrough in Hepatocellular Carcinoma. Liver Cancer 2016;5:1-7. [Crossref] [PubMed]



