
Near infrared fluorescence for image-guided surgery
Introduction
Surgery is among one of the most common treatments prescribed for acute and chronic diseases with as many as 40 million surgeries performed annually in the United States alone (1). Much of human surgery is performed without any image guidance where the surgeon has only direct visualization and palpation as their guide. Conventionally when image guidance is used it is in the form of X-ray fluoroscopy or ultrasound. Both of these imaging modalities provide tissue architecture information, but neither is amenable to targeted contrast agents that are desirable to provide tissue and disease specific contrast. Additionally, fluoroscopy exposes patients and the surgical team to ionizing radiation and ultrasound requires direct tissue contact for visualization, both significant disadvantages for intraoperative imaging (2). Of course additional medical imaging modalities including magnetic resonance imaging (MRI), computed tomography (CT), and the nuclear imaging modalities positron emission tomography (PET) and single photon emission computed tomography (SPECT) are readily available and are typically used to noninvasively assess the diseased tissue prior to the surgical procedure, but they have not found broad acceptance within the operating suite.
Optical imaging is gaining traction in the image-guided surgery field especially when coupled with near infrared (NIR) fluorophores, which can be targeted to help spare healthy tissue and remove diseased tissue through specific visualization. Imaging in the NIR region (700-900 nm) is advantageous because tissue absorbance, scattering, and autofluorescence are all at local minima, creating a black background upon which tissue specific contrast can be added. NIR fluorescent image-guided surgery affords surgeons numerous advantages including real-time, non-contact imaging, addition of contrast that does not change the look of the surgical field of view, and detection that does not require ionizing radiation. The advantages of NIR fluorescence have been recognized and many image-guided surgery systems are in preclinical and clinical development with a few systems even approved for limited clinical use (3,4).
Although NIR image-guided surgery has the potential for applications in numerous surgical procedures, the general focus of the research community has been on improvement of oncological surgery. This stems from the fact that surgery is currently the most effective, widely used cancer therapy, and cures about 45% of all cancer patients (5). The most accurate predictor of patient survival following surgical intervention is a resection with margins negative for cancer cells as determined by gold standard histopathology (6). Over the past 50 years, significant advances in surgical oncology have been made including minimally invasive surgery, preoperative assessment of disease through noninvasive imaging, and improved postoperative care. However, surgical cure rates have remained stagnant and little has changed about the intraoperative methods used to assess complete resection in the past 50 years (7). In addition to full removal of the tumor tissue, sentinel lymph node mapping is completed for some cancer types (specifically breast cancer and melanoma) as the standard of care, and as much normal tissue as possible is spared to decrease surgical morbidity. Each of these surgical goals would benefit from NIR fluorescent image-guided surgery to improve intraoperative visualization.
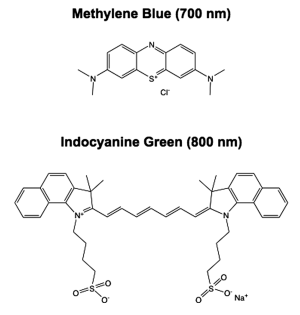
To provide the targeted visualization necessary for improved oncologic surgery, contrast agent development and clinical translation are necessary. Currently there are only two FDA approved NIR fluorescent contrast agents, indocyanine green (ICG) and methylene blue (MB) (Figure 1). Both ICG and MB are blood pool agents that are not inherently specific for any tumor tissue, and thus are not ideal fluorophores for oncologic image-guided surgery. However, because they are the only clinically approved NIR fluorophores, significant research and clinical trials have been completed with both fluorophores and many intraoperative uses have been found. Interestingly, although neither of the current NIR fluorophores are tumor-targeted agents, they have been found to highlight specific cancer types. ICG has been used for the widest variety of applications in NIR image-guided oncologic surgery to date (8). ICG has been clinically tested for sentinel lymph node mapping for a variety of cancers including breast, cervical, melanoma, vulvar, head and neck, and colorectal carcinoma (9-14). Clinical trials of perforator artery mapping with ICG for improved breast flap perfusion during reconstruction following mastectomy have also been completed (15,16). Additionally, ICG was found to specifically highlight tumors in the liver including hepatocellular carcinoma and colorectal liver metastases when systemically administered 1 day to 2 weeks prior to surgery. However, the specific contrast seen in these two cancer types is not because ICG is a tumor specific agent, but rather due to the pharmacokinetics and clearance route of ICG through the liver (17,18). MB has been shown to provide modest contrast in tumors of the pancreas including insulinoma in an animal model and a rare solitary fibrous tumor of the pancreas in a single patient (19,20). Although very preliminary results, it appears that MB may provide useful contrast to aid in resection of pancreatic carcinomas. MB and ICG are by no means optimized agents for NIR intraoperative imaging, but rather are blood pool agents that have found clinical use due to their FDA approval. To fully utilize NIR intraoperative imaging as a surgical tool in oncology optimized and targeted NIR fluorophores that can be translated to the clinical will be necessary. The following review outlines the chemical and biological properties necessary for an optimal small molecule NIR fluorophore and summarizes possible tumor and tissue targeting mechanisms. There is vast literature on nanoparticles and quantum dots for in vivo imaging, which has been reviewed recently, but is beyond the scope of the current review (21-24).
Chemical properties of NIR fluorophores
Excitation & Emission - The NIR region of the electromagnetic spectrum is advantageous for in vivo imaging. Between 700-900 nm the absorbance from the tissue chromophores including hemoglobin, water, and lipids are all at local minima. Additionally photon scatter at these wavelengths is also minimized (25). Tissue autofluorescence from endogenous chromophores including structural proteins (collagen and elastin), amino acids (tryptophan, tyrosine and phenylalanine), enzymes (FAD, NADH and NADPH), porphyrins (coproporphyrinogen, uroporphyrinogen and protoporphyrin), and lipids (phospholipids, lipofuscin and ceroid) are also minimal (26). Thus, optimal fluorophores will have excitation and emission wavelengths that fall within the NIR window between 700-900 nm to enable maximum photon penetration of the excitation light as well as a maximum number of photons returned from the fluorescent tissue to the detector of choice.
Extinction Coefficient & Quantum Yield - The extinction coefficient is an inherent property of the fluorophore, which determines how strongly it absorbs light according to the Beer-Lambert Law (A = clε, where A = measured absorbance, c = concentration of the fluorophore, l = path length of the measurement system, and ε = the extinction coefficient of the fluorophore). For fluorophores to provide adequate in vivo signal for visualization, the extinction coefficient must be relatively high, with the extinction coefficient of ICG and MB equal to 121,000 and 71,200 M-1cm-1, respectively (3). Quantum yield is also an inherent property of the fluorophore and is defined as the ratio of the number of photons emitted to the number of photons absorbed. The quantum yield is expressed as a percentage where typical small molecule NIR fluorophores usually have quantum yields between 10-20% in the in vivo environment. Of note, extinction coefficient and quantum yield can vary greatly depending on the solvent or environment, and thus for application to intraoperative imaging, these properties as well as determination of excitation and emission maxima should be characterized in serum to mimic the in vivo environment.
Solubility - Many NIR fluorophores, especially cyanine dyes, have low solubility in water based buffering systems. Additional chemical groups can be added to the base chemical structure to increase solubility. In the case of the cyanine base structure sulfonic acid groups are often added to increase solubility in water. Although it is most desirable for the NIR fluorophore to be dissolved in water based buffer, some fluorophores are too lipophilic and thus must be formulated for in vivo administration to enable circulation in the bloodstream. Drug formulation science is vast and will not be covered in the current review, although some potential methods to increase solubility through formulation include the addition of cremphor EL (a derivative of caster oil), cyclodextrin or polyethylene glycol of varied sizes and lengths (27-30). Of note, although addition of solubilizing groups to the base structure and formulation of the fluorophore can improve biocompatibility it can also significantly change biodistribution, pharmacokinetics, and clearance, therefore these properties should always be adequately characterized in the buffer or formulation used for in vivo administration.
Photostability - All small molecule fluorophores can undergo irreversible photodamage or photobleaching. Since during the surgical procedure, excitation light will be continually illuminating the surgical field of view, characterization of the photobleaching threshold for all NIR contrast agents to be utilized intraoperatively is of paramount importance. If the fluorophores are unstable, it is possible that photobleaching could be misinterpreted as lack of fluorescence contrast, which would give false negative results to the surgeon. Similar to other chemical properties, photostability is also solvent dependent and thus the best way to mimic the in vivo environment is to characterize the photobleaching threshold of NIR fluorophores in serum.
Biological properties of NIR fluorophores
Fluorophore Size & Clearance - It is well documented that the size and charge (as discussed in the following section) of systemically administered small molecules will determine their route of clearance from the body. In order to achieve signal to background ratio sufficient for imaging, clearance of all but the specifically bound fluorophore is desired. For systemically administered small molecules there are largely two routes of clearance from the body, renal (urine) and hepatic (bile to feces), where renal clearance is significantly faster than hepatic clearance. Studies on nanoparticles of varying size have shown that there is a size threshold above which all molecules are cleared hepatically. In general particles with a hydrodynamic diameter below 6 nm are cleared renally, while all larger particles are cleared hepatically (31). Although the preferred method of clearance will be dependent on the targeting method utilized, renal clearance is typically desired. If particles with hepatic clearance are to be utilized, the time between administration and imaging will often be sufficiently long that additional hospital visits will be required for contrast administration prior to the surgical procedure.
Charge & Charge Distribution - Studies by Choi et al. have demonstrated that biodistribution and clearance are significantly affected by charge and charge distribution across the molecule (24,27,28,31-33). Not only is the hydrodynamic diameter important for clearance, but the net charge across the molecule as well as charge distribution impact fluorophore clearance. Molecules with neutral, distributed net charge show the lowest background binding to normal organs and tissues. This has been demonstrated for both quantum dots and small molecules, with zwitterionic small molecule NIR fluorophores demonstrating the lowest background binding (34). This design parameter should be considered as new fluorophores and targeted fluorophores are developed for intraoperative use.
Small molecule NIR fluorophores
Chemical Base Structures with NIR Excitation & Emission - There are a limited number of known classes of small molecule NIR fluorophores including cyanines, phthalocyanines, porphyins, squaraine, borondipyrromethane (BODIPY), benzo[c]heterocycles, and xanthenes (Figure 2) (35,36). By far the most common class of NIR fluorophores are based on the cyanine chemical structure with varied length polymethine chains and dual aromatic nitrogen-containing heterocycles with varied substitutions to control excitation and emission wavelengths. In general, increasing the length of the polymethine chain causes bathochromatic shifts in excitation and emission wavelengths with pentamethine derivatives exhibiting ~700 nm fluorescence and heptamethine derivatives exhibiting ~800 nm fluorescence emission (3). Examples of regularly utilized cyanine fluorophores include ICG and the commercially available CyDyes (GE Healthcare Life Sciences). Phthalocyanine fluorophores are two dimensional 18π-electron aromatic chemical base structures, which have four bridged pyrrole subunits linked together through nitrogen atoms (37). Over 70 central metal ions can be incorporated into the phthalocyanine base structure and a variety of substituents can be added both at the periphery and axial positions which control the absorbance and emission wavelengths as well as physical properties of the fluorophores (38). Porphyins are cyclic chemical base structures with many similarities to the phthalocyanine chemical structures, where recent studies on porphyrin dimers have shown enhanced absorption and emission as well as photostability (39). The naturally occurring Protoporphyin IX (PpIX) is used for image-guided surgery of glioma resection. In randomized phase III clinical trials in Europe, image-guided surgery using PpIX fluorescence excited at 405 nm, with emission >650 nm was found to improve completeness of resection as assessed by postoperative MRI leading to increased progression-free survival of patients (40). Although not inherently a NIR fluorophore, PpIX image-guidance improves glioma resection and is currently in clinical trial in the United States (41). Squaraine (also termed squarylium dyes) are molecules with an electron deficient central four-membered (square) ring and resonance stabilized zwitterionic structure (36). Due to the planar nature of the chemical structure, solubility of these fluorophores has been difficult in the past, however recent work by Suzuki et al., has shown a squaraine fluorophore with increased solubility through the addition of multiple sulfonate moieties onto the squaraine base structure (42). In general squaraine fluorophores have strong absorption and extinction coefficients and exhibit excitation in the range of 650-800 nm with emission generally above 800 nm (37).
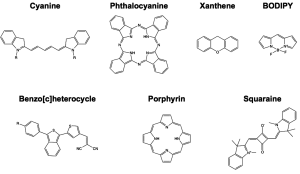
The remaining classes of fluorophores including BODIPY, benzo[c]heterocylces, and xanthenes have been synthesized from fluorophores which previously exhibited visible absorption and emission to shift their optical properties to the NIR. The BODIPY fluorophores have high stability and quantum yield although have traditionally had visible absorption and emission properties. Recently published studies of conjugation of aromatic rings to both ends of the BODIPY base structure have yielded promising fluorophores with increased quantum yield, higher extinction coefficient, improved photostability and NIR optical properties (43,44). Fluorophores synthesized using a unique donor-acceptor or push-pull type design that contain isobenzofuran or isothianaphthene moieties were recently published and found to have NIR optical properties (45). These unique benzo[c]heterocycle containing fluorophores were designed with physical properties that enabled passive diffusion across the blood brain barrier and were found to stain β-amyloid plaques, a characteristic structural signature of Alzheimer’s disease in the brain, and show promise for in vivo imaging applications (46,47). Recently published studies by Strongin et al., have demonstrated that conventionally visible xanthene fluorophores can be synthesized with an anion specific form that has NIR excitation and emission characteristics. These fluorophores are synthesized from the regioisomeric seminapthofluorone dye series and exhibit low molecular weight, aqueous solubility, and dual excitation and emission between their neutral (visible state) and anionic (NIR state) states (48).
Commercial Sources for NIR Fluorophores - Although there are seven known base classes of NIR fluorophores, there are a limited number of commercially available NIR fluorophores. Additionally, much of the chemical structure information is proprietary; however, it is known that commercially available NIR fluorophores are largely based on the cyanine chemical structure with specific modifications by each manufacturer. Clinical grade ICG and methylene blue can be purchased from Akorn and Taylor Pharmaceuticals, respectively. Chemical grade ICG and MB for laboratory work are also available through Sigma Aldrich. The most well known NIR fluorophores are the CyDyes manufactured by GE Healthcare Life Sciences. Their NIR fluorophores include the 700 nm fluorophore Cy5.5 and the 800 nm fluorophore Cy7 (49). Li-Cor Biosciences manufacturers the IRDye series with fluorophores with optical properties ranging from 650 to 800 nm, where IRDye 800CW has found the most widespread use in the in vivo preclinical literature to date (50). Invitrogen (formally Molecular Probes) manufactures a line of dyes known as the Alexa Fluor dyes with fluorophores with emission maximum ranging from 690 to 814 nm (51). Additional cyanine based fluorophores with slightly different chemical structure are sold by Pierce Protein Biology Products (distributed by Thermo Scientific) under the series name DyLight Fluors with absorption and emission wavelengths ranging from 650 to 800 nm (52). The performance of these fluorophores is similar to that of the Alexa Fluor series (unpublished observation), although they are less commonly published in the literature. More recently the Flamma Fluor series of dyes has been introduced by BioActs with emission wavelengths ranging from 700 to 800 nm (53). Lastly the ATTO series of fluorophores manufactured by ATTO-TEC and distributed by Sigma Aldrich have emission wavelengths up to 760 nm (54).
Although numerous commercial NIR fluorophores exist, the challenge of specific tumor targeting remains. In order to provide tissue or disease specific contrast each of the currently available fluorophores requires targeting to a tissue or disease specific ligand. Fortunately, each of the fluorophore series come as reactive fluorophores that can be readily conjugated to the protein or ligand of interest for specific in vivo targeting through lysine modification chemistry. Most of the commercially available experimental NIR fluorophores are available as N-hydroxysuccinimide (NHS) esters and isothiocyanates which can be used for fluorophore conjugation at a lysine residue or N-terminal amine (55).
Tissue & disease targeted strategies for NIR fluorophores
A fluorescent imaging agent that provides either normal or diseased tissue signal to background ratio is crucial for the utility of NIR fluorescent image-guided surgery. The possible methods to attain necessary signal to background ratio in the tissue of interest can be grouped into a few main categories and the advantages and disadvantages of each methodology are discussed as follows.
Enhanced Permeability & Retention (EPR) Effect - The EPR effect was discovered about 25 years ago to exist in tumor tissue. The generally leaky tumor vasculature is know to allow macromolecules roughly larger than 40 kDa to selectively leak out of tumor vessels and accumulate in the surrounding tumor tissue. This threshold is related to size in that long circulation times are necessary for passive targeting of tumor tissue using the EPR effect and thus significant tumor accumulation is only seen for molecules that are too large to be cleared from the body renally (56). Although the EPR effect does enable tumor accumulation of fluorophores, in general this passive targeting strategy does not provide high signal to noise ratio, is dependent upon the size of the particle and route of clearance, and does not inherently provide tumor-specific contrast (Figure 3A). In fact, recent work by Pogue et al., has demonstrated use of a dual fluorescent probe reporter system to calculate the amount of signal generated by passive and active targeting methodologies as the EPR effect is thought to confound quantification of actively targeted fluorescent contrast (57,58).
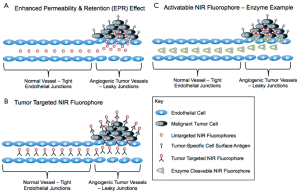
Targeted NIR Fluorophores - The most widely studied methodology for tumor-specific targeting of fluorophores is using a NIR fluorophore conjugated to a targeting sequence specific to cell surface markers present on cancer cells (Figure 3B). Targeting moieties that have been investigated range from small molecules, proteins, peptides, antibodies, and aptamers, all with advantages and disadvantages. In general, targeted contrast agents specific for the same targets used to treat cancer can be utilized to obtained adequate signal to background ratios within the diseased tissue. Often the targets of interest are cell surface biomarkers that are overexpressed on cancer cells as compared to normal cells. However, difficulties in obtaining adequate signal to background ratio for tumor detection abound from this strategy and are shared by the therapeutic world where off target binding of therapeutics results in normal tissue toxicity while off target binding of targeted fluorophores creates increased background signal, effectively lowering the visible signal to background ratio (2,59). Obviously this problem is not unique to image-guided surgery, but is a difficulty faced by the entire field of cancer biology, where biomarkers that enable adequate therapeutic ratio between normal and diseased tissue and high signal to background ratio for imaging purposes are few and far between. For imaging, this difficulty is compounded by the properties of the fluorophore attached to the targeting moiety, where the size and route of clearance of the compound can also affect the signal to background ratio, by increasing the background signal. However, with targeted fluorophores that are designed for optimal clearance from the body, tumor-specific targeting and imaging can provide higher signal to background ratio due to decreased background signal (34).
There are numerous preclinical studies using NIR fluorophores and tumor targeting moieties for all different cancer types, however to date only a single example of a tumor targeted fluorophore to guide oncologic surgery under image-guidance has been published. van Dam et al., describe a study where folate, which is specific for folate receptor α, was conjugated to the fluorophore fluorescein isothiocyanate (FITC). Previous studies of ovarian cancer demonstrated that folate receptor α is overexpressed in 90-95% of ovarian cancers and is not expressed on healthy ovarian cells, making it an ideal biomarker for ovarian carcinoma imaging (60). Due to clinical availability at the site of the clinical trial, the current study was conducted with the visible fluorophore (FITC) rather than a NIR fluorophore. The ability to visualize small, dispersed nodules of ovarian cancer scattered throughout the peritoneum demonstrate the utility of the technique with an appropriately targeted agent, even though the optical properties of the current fluorophore provide only very surface weighted imaging (60). The clinical example demonstrated in this work on a small number of ovarian cancer patients highlights the possibility for tumor-specific imaging with appropriate cancer biomarkers, where surgeons could imagine the ability to cut by color with the tumor specifically highlighted by targeted NIR fluorophores.
Activatable NIR Fluorophores - A second strategy to actively target tumor tissue is to utilize known differences in the microenvironment in order to provide tumor specific contrast. Enzyme activity can be significantly different in diseased tissues such as cancer as compared to healthy tissues, where probes activated by matrix metalloproteinases (MMPs) are one of the most well known examples. In these probes a peptide sequence recognized by a specific MMP was used to place two NIR fluorophores within close enough proximity to one another that their fluorescence emission signal was quenched. Through passive targeting and the EPR effect, the quenched probes accumulated in the tumor tissue where MMP activity was known to be higher than in benign tissues. The peptide sequence recognized by the MMP was cleaved and the NIR fluorophores were then separated by enough distance to emit fluorescence and be visualized (Figure 3C) (61-63). Optimization of the time following systemic administration of the quenched fluorophores prior to imaging is of utmost importance for these types of probes as they are not specifically targeted to the tumor tissue, and once the fluorophores are cleaved and actively emitting photons they can diffuse from the tumor and emit fluorescence elsewhere within the body. Additional tumor specific enzymes have also been targeted using a similar strategy of a small peptide that is recognized by the enzyme of interest that holds the fluorophores within appropriate distance to quench one another until enzymatically cleaved (64,65).
Additional differences in the cancer microenvironment as compared to the microenvironment of normal tissue have been exploited to create tumor specific contrast. One example uses fluorophores that are pH sensitive and can detect the known slightly acidic pH present in the tumor microenvironment (66-68). Some recent examples of pH sensitive probes have been published and found to provide tumor-specific contrast in preclinical tumor models (69,70). There are many additional differences that are currently being explored between malignant and benign tissue microenvironments, however no clinical examples of these activatable NIR fluorophores for image-guided surgery currently exist.
Tissue-specific NIR Fluorophores - In addition to targeting diseased tissues such as tumors, targeting crucial normal tissue structures to be avoided during oncologic surgery such as blood vessels and nerve tissue is also of significant importance. Fortunately, clinically approved blood pool agents such as ICG can be utilized for vascular imaging and have shown clinical utility for this application in perforator artery mapping for tram flap breast reconstruction following mastectomy in first in human studies (15). Developing a NIR nerve-specific fluorophore is also of significant clinical interest as nerve tissue can be difficult to visualize during surgery because the small, translucent structures are typically protected deep within the tissue. Additionally, some surgical sites are precariously close to critical nerves such as the cavernous nerve, which is often damaged during prostatectomy. Development of a NIR nerve-specific contrast agent would significantly improve visualization and decrease surgical morbidity for patients. Currently, two strategies to target nerve tissue have been published. One strategy utilized nerve-targeting peptides that are specific for the epinerium surrounding the nerve tissue and were conjugated to Cy5 (71). A second strategy used small molecule fluorophores specific for nerve tissue and provided homogenous signal across the nerve bundles when administered systemically. Currently published studies demonstrate a class of small molecule that provide nerve-specific contrast, but do not have optimized optical properties for image-guided surgery (29,30).
Conclusions & the future of NIR image-guided surgery
NIR image-guided surgery shows significant promise to improve surgical outcomes for patients especially in the realm of oncologic surgery. A number of NIR image-guided surgical systems have been designed and tested with some approved for clinical use. In order to make NIR image-guided surgery a reality for patients and surgeons, NIR fluorophores for clinical use are necessary. Specifically targeted agents for diseased tissues such as tumors are desirable to ensure full resection of the tumor at the time of surgery with margins clear of cancer cells. Additionally, tissue specific contrast agents to highlight normal tissues such as nerves to be spared during surgery are desirable. Numerous NIR fluorophores and tissue targeting strategies have been developed and tested preclinically, but in order for this technology to benefit patients the difficult path of clinical translation will need to be navigated.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Burke S, Shorten GD. When pain after surgery doesn’t go away. Biochem Soc Trans 2009;37:318-22. [PubMed]
- Frangioni JV. New technologies for human cancer imaging. J Clin Oncol 2008;26:4012-21. [PubMed]
- Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging 2010;9:237-55. [PubMed]
- Keereweer S, Kerrebijn JD, van Driel PB, et al. Optical image-guided surgery--where do we stand? Mol Imaging Biol 2011;13:199-207. [PubMed]
- De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat 2003;2:553-62. [PubMed]
- Lim MC, Seo SS, Kang S, et al. Intraoperative image-guided surgery for ovarian cancer. Quant Imaging Med Surg 2012;2:114-7.
- Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. Annu Rev Med 2010;61:359-73. [PubMed]
- Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 2011;104:323-32. [PubMed]
- Hutteman M, Choi HS, Mieog JS, et al. Clinical translation of ex vivo sentinel lymph node mapping for colorectal cancer using invisible near-infrared fluorescence light. Ann Surg Oncol 2011;18:1006-14. [PubMed]
- Hutteman M, Mieog JS, van der Vorst JR, et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat 2011;127:163-70. [PubMed]
- Mieog JS, Troyan SL, Hutteman M, et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol 2011;18:2483-91. [PubMed]
- Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 2009;16:2943-52. [PubMed]
- Hutteman M, van der Vorst JR, Gaarenstroom KN, et al. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am J Obstet Gynecol 2012;206:89.e1-5.
- Schaafsma BE, van der Vorst JR, Gaarenstroom KN, et al. Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol Oncol 2012;127:126-30. [PubMed]
- Lee BT, Hutteman M, Gioux S, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg 2010;126:1472-81. [PubMed]
- Lee BT, Matsui A, Hutteman M, et al. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg 2010;26:59-65. [PubMed]
- Ishizawa T, Bandai Y, Hasegawa K, et al. Fluorescent cholangiography during laparoscopic cholecystectomy: indocyanine green or new fluorescent agents? World J Surg 2010;34:2505-6. [PubMed]
- Verbeek FP, van der Vorst JR, Schaafsma BE, et al. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci 2012. [Epub ahead of print].
- van Vorst JR, Vahrmeijer AL, Hutteman M, et al. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J Gastrointest Surg 2012;4:180-4. [PubMed]
- Winer JH, Choi HS, Gibbs-Strauss SL, et al. Intraoperative localization of insulinoma and normal pancreas using invisible near-infrared fluorescent light. Ann Surg Oncol 2010;17:1094-100. [PubMed]
- He X, Wang K, Cheng Z.
In vivo near-infrared fluorescence imaging of cancer with nanoparticle-based probes. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010;2:349-66. [PubMed] - Altinoğlu EI, Adair JH. Near infrared imaging with nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010;2:461-77. [PubMed]
- He X, Gao J, Gambhir SS, et al. Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges. Trends Mol Med 2010;16:574-83. [PubMed]
- Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging 2010;9:291-310. [PubMed]
- Frangioni JV.
In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626-34. [PubMed] - Ramanujam N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000;2:89-117. [PubMed]
- Choi HS, Ipe BI, Misra P, et al. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett 2009;9:2354-9. [PubMed]
- Choi HS, Liu W, Liu F, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol 2010;5:42-7. [PubMed]
- Cotero VE, Siclovan T, Zhang R, et al. Intraoperative Fluorescence Imaging of Peripheral and Central Nerves Through a Myelin-Selective Contrast Agent. Mol Imaging Biol 2012; [Epub ahead of print]. [PubMed]
- Gibbs-Strauss SL, Nasr KA, Fish KM, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging 2011;10:91-101. [PubMed]
- Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165-70. [PubMed]
- Choi HS, Ashitate Y, Lee JH, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol 2010;28:1300-3. [PubMed]
- Liu W, Choi HS, Zimmer JP, et al. Compact cysteine-coated CdSe(ZnCdS) quantum dots for in vivo applications. J Am Chem Soc 2007;129:14530-1. [PubMed]
- Choi HS, Nasr K, Alyabyev S, et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem Int Ed Engl 2011;50:6258-63. [PubMed]
- Lavis LD, Raines RT. Bright ideas for chemical biology. ACS Chem Biol 2008;3:142-55. [PubMed]
- Escobedo JO, Rusin O, Lim S, et al. NIR dyes for bioimaging applications. Curr Opin Chem Biol 2010;14:64-70. [PubMed]
- Luo S, Zhang E, Su Y, et al. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011;32:7127-38. [PubMed]
- de la Torre G, Claessens CG, Torres T. Phthalocyanines: old dyes, new materials. Putting color in nanotechnology. Chem Commun (Camb) 2007;20:2000-15. [PubMed]
- Kuimova MK, Collins HA, Balaz M, et al. Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy. Org Biomol Chem 2009;7:889-96. [PubMed]
- Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392-401. [PubMed]
- Roberts DW, Valdés PA, Harris BT, et al. Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic acid). Neurosurg Clin N Am 2012;23:371-7. [PubMed]
- Umezawa K, Citterio D, Suzuki K. Water-soluble NIR fluorescent probes based on squaraine and their application for protein labeling. Anal Sci 2008;24:213-7. [PubMed]
- Umezawa K, Matsui A, Nakamura Y, et al. Bright, color-tunable fluorescent dyes in the Vis/NIR region: establishment of new “tailor-made” multicolor fluorophores based on borondipyrromethene. Chemistry 2009;15:1096-106. [PubMed]
- Umezawa K, Nakamura Y, Makino H, et al. Bright, color-tunable fluorescent dyes in the visible-near-infrared region. J Am Chem Soc 2008;130:1550-1. [PubMed]
- Meek ST, Nesterov EE, Swager TM. Near-infrared fluorophores containing benzo[c]heterocycle subunits. Org Lett 2008;10:2991-3. [PubMed]
- Raymond SB, Skoch J, Hills ID, et al. Smart optical probes for near-infrared fluorescence imaging of Alzheimer’s disease pathology. Eur J Nucl Med Mol Imaging 2008;35:S93-8. [PubMed]
- Nesterov EE, Skoch J, Hyman BT, et al.
In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers. Angew Chem Int Ed Engl 2005;44:5452-6. [PubMed] - Yang Y, Lowry M, Xu X, et al. Seminaphthofluorones are a family of water-soluble, low molecular weight, NIR-emitting fluorophores. Proc Natl Acad Sci U S A 2008;105:8829-34. [PubMed]
- Sciences GHL. CyDye- Available online: http://www.gelifesciences.com/webapp/wcs/stores/servlet/catalog/en/GELifeSciences-us/brands/cydye/
- Biosciences L-C. IRDye Infrared Dye - Available online: http://www.licor.com/bio/products/reagents/irdye/index.jsp 2012
- Technologies I-L. Alexa Fluor Dyes - Available online: http://www.invitrogen.com/site/us/en/home/brands/Molecular-Probes/Key-Molecular-Probes-Products/alexa-fluor/Alexa-Fluor-Dyes-Across-the-Spectrum.html 2012
- Products TS-PPB. DyLight Fluor - Available online: http://www.piercenet.com/browse.cfm?fldID=294EFD98-4461-4352-A046-50B522F52952 2012
- BioActs. Flamma Series - Available online: http://ebioacts.com/02_product/01_flamma_p.php 2012
- ATTO-TEC. ATTO Fluorescent Labels - Available online: https://http://www.atto-tec.com/attotecshop/index.php?cat=c1_Fluorescent-Labels.html&XTCsid=e58eeg8rc9mimvccsih9r6aii7 2012
- Stephanopoulos N, Francis MB. Choosing an effective protein bioconjugation strategy. Nat Chem Biol 2011;7:876-84. [PubMed]
- Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 2011;63:136-51. [PubMed]
- Tichauer KM, Samkoe KS, Sexton KJ, et al. In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging. Mol Imaging Biol 2012;14:584-92. [PubMed]
- Tichauer KM, Samkoe KS, Sexton KJ, et al. Improved tumor contrast achieved by single time point dual-reporter fluorescence imaging. J Biomed Opt 2012;17:066001. [PubMed]
- Frangioni JV. The problem is background, not signal. Mol Imaging 2009;8:303-4. [PubMed]
- van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 2011;17:1315-9. [PubMed]
- Bremer C, Bredow S, Mahmood U, et al. Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology 2001;221:523-9. [PubMed]
- Bremer C, Tung CH, Bogdanov A Jr, et al. Imaging of differential protease expression in breast cancers for detection of aggressive tumor phenotypes. Radiology 2002;222:814-8. [PubMed]
- Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med 2001;7:743-8. [PubMed]
- Ogawa M, Kosaka N, Longmire MR, et al. Fluorophore-quencher based activatable targeted optical probes for detecting in vivo cancer metastases. Mol Pharm 2009;6:386-95. [PubMed]
- Ogawa M, Kosaka N, Choyke PL, et al. H-type dimer formation of fluorophores: a mechanism for activatable, in vivo optical molecular imaging. ACS Chem Biol 2009;4:535-46. [PubMed]
- Huber V, De Milito A, Harguindey S, et al. Proton dynamics in cancer. J Transl Med 2010;8:57. [PubMed]
- Gillies RJ, Raghunand N, Garcia-Martin ML, et al. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag 2004;23:57-64. [PubMed]
- Chiche J, Brahimi-Horn MC, Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 2010;14:771-94. [PubMed]
- Mathejczyk JE, Pauli J, Dullin C, et al. High-sensitivity detection of breast tumors in vivo by use of a pH-sensitive near-infrared fluorescence probe. J Biomed Opt 2012;17:076028. [PubMed]
- Zhang Z, Achilefu S. Design, synthesis and evaluation of near-infrared fluorescent pH indicators in a physiologically relevant range. Chem Commun (Camb) 2005;47:5887-9. [PubMed]
- Whitney MA, Crisp JL, Nguyen LT, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol 2011;29:352-6. [PubMed]


