
IgG4-related inflammatory pseudotumor involving the temporal bone disguised as meningioma: a case description and literature analysis
Introduction
In 2003, immunoglobulin G4 (IgG4)-related disease (IgG4-RD) was identified as a unique disease (1). Fibroinflammatory lesions caused by IgG4-RD can mimic a tumor and affect nearly any organ, including the lacrimal glands, orbits, pancreas, bile ducts, major salivary glands, renal tubules, bones, lungs, aorta, pachymeninges, and thyroid gland (2,3). IgG4-RD is characterized pathologically by the presence of lymphoplasmacytic infiltration, storiform fibrosis, obliterative phlebitis, and prominent IgG4-positive plasma cell infiltrates (4). Nearly 20% of patients with IgG4-RD often have normal concentrations of serum IgG4 (5). A wide range of IgG4-RD lesions has been found to affect the temporal bone, including space-occupying lesions that cause cranial nerve palsies and locally invasive lesions that cause bony destruction (6). IgG4-RD lesions must also be differentiated from benign and malignant tumors involving the temporal bone, such as hemangioma, meningioma, cancer, and lymphoma. Furthermore, IgG4-related neurological disease symptoms frequently resemble tumors, infections, and other inflammatory disorders. Here, we present a rare case of an IgG4-related inflammatory pseudotumor in the temporal bone that resembled a meningioma and discuss the relevant literature for reviewing the symptoms, treatment, and prognosis of the disease.
Case presentation
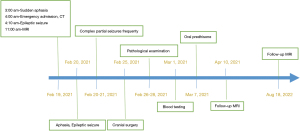
A 57-year-old man with a 4-hour history of aphasia was admitted to a local hospital. The patient was drowsy and mute, but he could open his eyes, move his limbs, and respond to some movement commands. The muscle strength of the right leg was grade 3–4, and that of the left leg was grade 5. There were no symptoms or signs of meningeal irritation. At 10 minutes after admission, the patient began to experience epileptic seizures, which were controlled by intravenous diazepam. Over the following 3 days, the patient experienced repeated, intermittent partial seizures, for which the treatment of intravenous diazepam became ineffective.
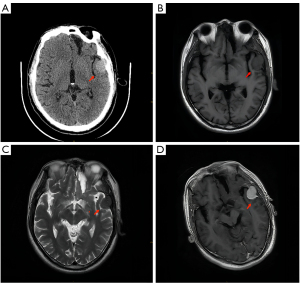
Computed tomography (CT) of the patient’s head revealed a circular shape of a slightly uniform density with a clear boundary. The bone nearby was destroyed (Figure 1A). Magnetic resonance imaging (MRI) of the patient’s head showed the shadow of a round, well-defined mass on the left temporal part, with a size of approximately 2.7 cm × 2.1 cm. Both the T1-weighted image (T1WI) and T2-weighted image (T2WI) of the mass were isointense (Figure 1B,1C), while the apparent diffusion coefficient (ADC) was hypointense. The adjacent brain parenchyma was compressed and could be distinguished from the adjacent brain parenchyma by a curved cerebrospinal fluid shadow. The mass was very close to the left temporal bone, and part of the adjacent bone was found to be destroyed and absorbed. After gadolinium contrast enhancement, the mass showed obvious uniform enhancement with a clear boundary and a meningeal tail sign (Figure 1D). The combined CT and MRI findings indicated meningioma, which needed to be differentiated from Langerhans histiocytosis and myeloma.
The patient underwent a craniotomy. During surgery, the space-occupying lesion was located on the left temporal floor, growing across the dura mater into the epidural temporal floor and subdural space. The left temporal pole and the local temporal bone were significantly compressed. The blood supply for the tumor came from a branch of the middle meningeal artery at the temporal base. The tumor resembled a circular mass with a pale and solid cut surface and clear borders.
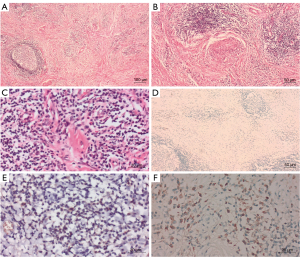
Histopathology revealed that the tumor was an inflammatory pseudotumor characterized by sclerosing fibrosis, including lymphocytes and plasma cells. A large amount of lymphoplasmacytic infiltrates that formed lymph follicles were observed, along with thick collagen fibers arranged in a storiform pattern, obliterative phlebitis, and plasma cells invading the tissues (Figure 2A-2C). Hematoxylin and eosin staining revealed that the fusiform cells of the fibrous tissue had been, in all probability, mistaken for meningioma cells. Immunohistochemistry revealed that the fusiform cells were negative for pan-cytokeratin (pCK), epithelial membrane antigen (EMA), calretinin (Cr), and progesterone receptor (PR), suggesting meningioma could be ruled out (Figure 2D). The lymphocytes were positive for cluster of differentiation 20 (CD20) and CD3. Numerous plasma cells were positive for CD138 (Figure 2E). Moreover, 40% of the plasma cells stained positive for IgG4. There were more than 60 IgG4-positive cells per high-power field (HPF; Figure 2F). Based on these findings, the pathologic diagnosis was IgG4-RD.
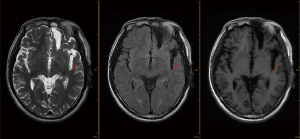
Blood samples were also collected for testing. The samples revealed a normallevel of total IgG and IgG4 in the blood serum. Ultrasound examinations of the salivary glands and pancreaticobiliary system after the initial examination did not reveal any abnormalities. After the diagnosis of IgG4-RD, the patient was prescribed 60 mg of prednisone in order to avoid any emergency events caused by intracranial tumor recurrence. After a month of treatment, MRI revealed that the lesions had disappeared, and the patient had no symptoms of epileptic seizures. The patient was treated with 5 mg/d prednisone maintenance for one year. After one and a half years, the MRI results showed no tumor recurrence, and no IgG4-related lesions were found in other organs (Figures 3,4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
IgG4-RD is a chronic, fibrotic, immune-mediated disorder characterized by the formation of tumor-like masses in multiple organs. CD4+ cytotoxic T lymphocytes (CTLs) activated by B cells dominate the immune cell infiltration in IgG4-RD. In IgG4-RD, macrophages, activated B cells, CD4+ CTLs, and fibroblasts are likely all implicated in creating inflammatory masses consisting of immune cells and fibrotic tissue (7). Friedrich et al. (8) looked into the crosstalk between T cells that happens when CC-chemokine ligand 5 (CCL5), which is expressed on CD8 effector memory (TEM), binds to C-C chemokine receptor type 4/5 (CCR4/5)-expressing T cells. The signals of T-cell-T-cell crosstalk could help recruit helper and cytotoxic CD4+ T cells, which have been shown to cause inflammation in IgG4-RD (8). IgG4-RD does not generate a fever and seldom leads to rapid organ failure; therefore, patients’ symptoms and indicators of illness may go unnoticed by medical professionals (9). It is common for IgG4-RD to result in organ enlargement, mimicking tumors. Furthermore, approximately 3–30% of patients with IgG4-RD have normal serum IgG4 concentrations (10,11). Therefore, elevated IgG4 concentrations are neither necessary nor sufficient to diagnose IgG4-RD (10).
In 2019, the American College of Rheumatology proposed guidelines for detecting IgG4-RD based on a range of characteristic symptoms and the lack of another reasonable diagnosis (5). Signature histological findings included lymphoplasmacytic infiltration, storiform fibroids, and obliterative phlebitis. IgG4-RD is diagnosed if the ratio of IgG4+ to IgG+ is greater than 40% and there are 10 IgG4+ plasma cells per HPF (5). In the present case, we diagnosed an IgG4-related inflammatory pseudotumor based on pathological features, namely sclerosing fibrosis and lymphocytes, as well as IgG4-positive plasma cell infiltration into the tissues.
IgG4-RD can occur in all parts of the body. Recent reports indicate that the nervous system can also be involved, mainly the skull and meninges (12-14). A comprehensive analysis of 184 cases of IgG4-RD in the skull base and skull found that the dura mater and meninges were the most common sites involved (32%) (15). Up to now, 22 cases of IgG4-RD involving the temporal bone have been reported (Table 1) (16-32). Meningeal IgG4-RD symptoms typically occur in isolation with no other organ involvement when serum IgG4 concentrations are normal or barely elevated (33). The most frequent symptom of IgG4-RD is hearing loss, while seizures are rare (6,9). In the present case, seizures were the only clinical symptom. In this case, it was the temporal bone and meninges that were involved, which was different from the most common involvement of the mastoid process in existing reports (6). The lesion was observed to be completely extradural. In addition, the lesion was surgically removed at the early stage, and the diagnosis was confirmed by pathology. The T1-weighted MRI showed that 75% of the lesion appeared isointense, while T2-weighted MRI showed 62.5% hypointensity, but all other cases reported involved positive enhancement (34). This case was slightly different from those in the literature because the signals on T1- and T2-weighted images were isointense, which was significantly strengthened after enhancement.
Table 1
| Case No. | Authors | Age, year | Sex | Symptoms |
|---|---|---|---|---|
| 1 | Masterson et al. [2010] (16) | 58 | F | Tinnitus, vertigo, and sensorineural hearing loss |
| 2 | Schiffenbauer et al. [2012] (17) | 50 | F | Otalgia, otitis media, and CN VII palsy |
| 3 | Moss et al. [2012] (18) | 36 | F | Headache, vision loss, CN VI palsy, diplopia |
| 4 | Bittencourt et al. [2013] (19) | 28 | M | Headache, otalgia, hearing loss, tinnitus |
| 5 | Nishijima et al. [2013] (20) | 66 | F | Hearing loss, facial numbness, headache, diplopia, ptosis, and fullness in the ears |
| 6 | Wang et al. [2015] (21) | 38 | M | Right temporal headache, right catarrhal otitis media |
| 7 | Cain et al. [2014] (22) | 66 | M | Headache, vertigo, hearing loss |
| 8 | Liu et al. [2015] (23) | 71 | M | Hearing loss, tinnitus, upper neck pain |
| 9 | Li et al. [2017] (24) | 52 | M | Hearing loss on both sides, vestibular dysfunction, and otalgia |
| 10 | Wick et al. [2016] (25) | 61 | F | Otalgia, hearing impairment, facial weakness, diplopia CN VI and CN VII palsies |
| 11 | Deshpande et al. [2016] (26) | 43 | F | Pulsatile tinnitus, hearing loss, mastoiditis |
| 12 | Deshpande et al. [2016] (26) | 52 | F | Headache, hearing loss, otalgia, CN VII, weakness, mastoiditis |
| 13 | Deshpande et al. [2016] (26) | 50 | F | Paresis of CN VII; mastoiditis, serous otitis media, hearing loss |
| 14 | Vuncannon et al. [2017] (27) | 35 | F | Hearing loss, otalgia, tinnitus, dizziness |
| 15 | Chowsilpa et al. [2019] (28) | 19 | F | Otalgia, lateral rectus palsy, headache, and hearing loss on the left |
| 16 | Cheng et al. [2019] (29) | 54 | F | Left ear otalgia, tinnitus, and hearing loss |
| 17* | Melenotte et al. [2019] (30) | 58 | F | Hallucination, aphasia, seizures, cognitive decline |
| 18 | Detiger et al. [2020] (31) | 73 | M | Otitis media with hearing loss, left otalgia, and left jaw pain |
| 19 | Marinelli et al. [2020] (32) | 55 | M | Headache, fatigue, weight loss, diplopia |
| 20 | Marinelli et al. [2020] (32) | 66 | F | Headache, facial pain, vertigo, sensorineural, hearing loss, diplopia |
| 21 | Marinelli et al. [2020] (32) | 59 | F | Headache, proptosis, sensorineural hearing loss |
| 22 | Marinelli et al. [2020] (32) | 65 | M | Vertigo, tinnitus, conductive hearing loss |
*, case with the symptom of seizures. IgG4, immunoglobulin G4; CN, cranial nerve; M, male; F, female.
The results of CT and MRI in the present case were confusing. The findings were very similar to meningioma but differed from those of benign meningiomas, which are generally located under the inner plate with hyperplastic and sclerotic changes to the bone. In our patient, compression and absorption of adjacent bone were evident, suggesting a diagnosis of another benign tumor. Available studies suggest that if a bulging mass is attached to the dura mater, meningioma should be considered, which is typically diagnosed by meningeal biopsy (35). In terms of manifestation of the temporal bone, the most common differential diagnosis of the IgG-RD includes granulomatosis with polyangiitis (GPA), natural killer (NK)/T lymphoma, mycosis infection, and Langerhans histiocytosis or myeloma, All diseases that can manifest in the temporal bone and exhibit nonspecific, inflammatory imaging appearances (36). Serologic tests, including the C-anti-neutrophil cytoplasm antibodies (C-ANCA), P-anti-neutrophil cytoplasm antibodies (P-ANCA), soluble interleukin 2 receptor, and beta-D glucan, could assist in differential diagnosis. Most patients with IgG4-RD respond to glucocorticoid treatment, with a 93% overall response rate and a 66% complete response rate (37). Rituximab is a monoclonal anti-CD20 antibody that is a promising method for treating glucocorticoid-refractory diseases (38). However, due to its high cost and possible side effects, rituximab remains primarily a second-line treatment in clinical practice. Nevertheless, it should be considered in advance for diseases that endanger life or organs.
Conclusions
The present patient had an IgG4-associated inflammatory pseudotumor in the temporal region with similar neuroradiological findings to meningioma. Temporal bone IgG4-RD is a rare disease. However, when a patient suffers seizures and image findings of the temporal bone lead clinicians to suspect meningioma, IgG4-RD should be a differential diagnosis. Glucocorticoids are usually the first choice of treatment for IgG4-RD. With increasing knowledge of the pathophysiology of IgG4-RD, new therapeutic approaches may become available in the near future.
Acknowledgments
We would like to thank the members and staff of the Department of Pathology and Radiology of the Third People’s Hospital of Chengdu Pujiang Hospital who contributed to this manuscript.
Funding: This study was supported by the Chengdu Medical Scientific Research Project in 2022 (No. 2022496).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-787/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012;366:539-51. [Crossref] [PubMed]
- Sekiguchi H, Horie R, Kanai M, Suzuki R, Yi ES, Ryu JH. IgG4-Related Disease: Retrospective Analysis of One Hundred Sixty-Six Patients. Arthritis Rheumatol 2016;68:2290-9. [Crossref] [PubMed]
- Kuroda N, Nao T, Fukuhara H, Karashima T, Inoue K, Taniguchi Y, Takeuchi M, Zen Y, Sato Y, Notohara K, Yoshino T. IgG4-related renal disease: clinical and pathological characteristics. Int J Clin Exp Pathol 2014;7:6379-85. [PubMed]
- Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet 2015;385:1460-71. [Crossref] [PubMed]
- Wallace ZS, Naden RP, Chari S, Choi H, Della-Torre E, Dicaire JF, et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-Related Disease. Arthritis Rheumatol 2020;72:7-19. [Crossref] [PubMed]
- Oochit KK, Wong YY, Mihuna A, Oliwa A, Kontorinis G. IgG4-Related Sclerosing Disease of the Temporal Bone: A Systematic Review. Otol Neurotol 2022;43:856-63. [Crossref] [PubMed]
- Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol 2020;16:702-14. [Crossref] [PubMed]
- Friedrich M, Kehl N, Engelke N, Kraus J, Lindner K, Münch P, Mildenberger I, Groden C, Gass A, Etminan N, Fatar M, von Deimling A, Reuss D, Platten M, Bunse L. Intrathecal activation of CD8+ memory T cells in IgG4-related disease of the brain parenchyma. EMBO Mol Med 2021;13:e13953. [Crossref] [PubMed]
- Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, Stone JH. IgG4-Related Disease: Clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol 2015;67:2466-75. [Crossref] [PubMed]
- Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14-8. [Crossref] [PubMed]
- Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci 2014;21:43-50. [Crossref] [PubMed]
- Deshpande V. IgG4 related disease of the head and neck. Head Neck Pathol 2015;9:24-31. [Crossref] [PubMed]
- Ferry JA, Deshpande V. IgG4-related disease in the head and neck. Semin Diagn Pathol 2012;29:235-44. [Crossref] [PubMed]
- Toyoda K, Oba H, Kutomi K, Furui S, Oohara A, Mori H, Sakurai K, Tsuchiya K, Kan S, Numaguchi Y. MR imaging of IgG4-related disease in the head and neck and brain. AJNR Am J Neuroradiol 2012;33:2136-9. [Crossref] [PubMed]
- Cler SJ, Sharifai N, Baker B, Dowling JL, Pipkorn P, Yaeger L, Clifford DB, Dahiya S, Chicoine MR. IgG4-Related Disease of the Skull and Skull Base-A Systematic Review and Report of Two Cases. World Neurosurg 2021;150:179-196.e1. [Crossref] [PubMed]
- Masterson L, Del Pero MM, Donnelly N, Moffat DA, Rytina E. Immunoglobulin G4 related systemic sclerosing disease involving the temporal bone. J Laryngol Otol 2010;124:1106-10. [Crossref] [PubMed]
- Schiffenbauer AI, Wahl C, Pittaluga S, Jaffe ES, Hoffman R, Khosroshahi A, Stone JH, Deshpande V, Gahl WA, Gill F. IgG4-related disease presenting as recurrent mastoiditis. Laryngoscope 2012;122:681-4. [Crossref] [PubMed]
- Moss HE, Mejico LJ, de la Roza G, Coyne TM, Galetta SL, Liu GT. IgG4-related inflammatory pseudotumor of the central nervous system responsive to mycophenolate mofetil. J Neurol Sci 2012;318:31-5. [Crossref] [PubMed]
- Bittencourt AG, Pereira LV, Cabral F Jr, Halang Fde S, Gonçalves Mde C, Bento RF. IgG4-related sclerosing disease of the temporal bone. Otol Neurotol 2013;34:e20-1. [Crossref] [PubMed]
- Nishijima H, Arai A, Kon T, Funamizu Y, Ueno T, Haga R, Miki Y, Kimura T, Suzuki C, Tomiyama M, Kusumi T, Minato H, Baba M. Intracranial immunoglobulin G4-related disease successfully treated by steroid and oral cyclophosphamide: A case report. Neurol Clin Neurosci 2013;1:38-40. [Crossref]
- Wang J, Sun Z, Zhuo S, Wang K. Sigmoid sinus occlusion infiltrated by inflammatory myofibroblastic tumor from mastoid. Head Neck 2015;37:E4-7. [Crossref] [PubMed]
- Cain RB, Colby TV, Balan V, Patel NP, Lal D. Perplexing lesions of the sinonasal cavity and skull base: IgG4-related and similar inflammatory diseases. Otolaryngol Head Neck Surg 2014;151:496-502. [Crossref] [PubMed]
- Liu J, Zhang B, Sun H, Han J, Yang D. Immunoglobulin G4-related disease in the skull base mimicking nasopharyngeal carcinoma. J Craniofac Surg 2015;26:e144-5. [Crossref] [PubMed]
- Li L, Ward B, Cocks M, Kheradmand A, Francis HW. IgG4-Related Disease of Bilateral Temporal Bones. Ann Otol Rhinol Laryngol 2017;126:236-40. [Crossref] [PubMed]
- Wick CC, Zachariah J, Manjila S, Brown WC, Malla P, Katirji B, Cohen M, Megerian CA. IgG4-related disease causing facial nerve and optic nerve palsies: Case report and literature review. Am J Otolaryngol 2016;37:567-71. [Crossref] [PubMed]
- Deshpande V, Zane NA, Kraft S, Stone JH, Faquin WC. Recurrent Mastoiditis Mimics IgG4 Related Disease: A Potential Diagnostic Pitfall. Head Neck Pathol 2016;10:314-20. [Crossref] [PubMed]
- Vuncannon JR, Panella NJ, Magliocca KR, Mattox DE. Diagnostic Challenges in a Case of IgG4-RD Affecting the Temporal Bone. Ann Otol Rhinol Laryngol 2017;126:241-4. [Crossref] [PubMed]
- Chowsilpa S, Chowsilpa S, Teeranoraseth T, Roongrotwattanasiri K. Temporal bone involvement of IgG4-related disease: a rare condition misleading to petrous apicitis causing lateral rectus palsy. BMJ Case Rep 2019;12:e228550. [Crossref] [PubMed]
- Cheng X, Shu Y, Chen B. A solely ear-involved IgG4-related sclerosing disease with two-years following-up. Eur Ann Otorhinolaryngol Head Neck Dis 2019;136:401-3. [Crossref] [PubMed]
- Melenotte C, Seguier J, Ebbo M, Kaphan E, Bernit E, Saillier L, et al. Clinical presentation, treatment and outcome of IgG4-related pachymeningitis: From a national case registry and literature review. Semin Arthritis Rheum 2019;49:430-7. [Crossref] [PubMed]
- Detiger SE, Karim F, Monserez D, Verdijk R, van Hagen M, Paridaens D, van Laar J. IgG4-Related Disease of Skull Base: Case Series of 3 Patients with Headache. World Neurosurg 2020;134:536-9. [Crossref] [PubMed]
- Marinelli JP, Marvisi C, Vaglio A, Peters PA, Dowling EM, Palumbo AA, Lane JI, Appelbaum EN, Sweeney AD, Carlson ML. Manifestations of Skull Base IgG4-Related Disease: A Multi-Institutional Study. Laryngoscope 2020;130:2574-80. [Crossref] [PubMed]
- AbdelRazek MA, Venna N, Stone JH. IgG4-related disease of the central and peripheral nervous systems. Lancet Neurol 2018;17:183-92. [Crossref] [PubMed]
- Spinazzi EF, Desai SV, Fang CH, Jyung RW, Liu JK, Baredes S, Eloy JA. Lateral skull base Inflammatory pseudotumor: A systematic review. Laryngoscope 2015;125:2593-600. [Crossref] [PubMed]
- Mehta SH, Switzer JA, Biddinger P, Rojiani AM. IgG4-related leptomeningitis: a reversible cause of rapidly progressive cognitive decline. Neurology 2014;82:540-2. [Crossref] [PubMed]
- Sireesha Y, Uppin MS, Ganti S, Alugolu R, Mudumba VS, Bhattacharjee S, Neeharika ML, Bastia J, Kanikannan MA. A Series of Biopsy-proven Patients with Immunoglobulin G4-related Neurological Disease. Ann Indian Acad Neurol 2019;22:73-8. [Crossref] [PubMed]
- Masaki Y, Matsui S, Saeki T, Tsuboi H, Hirata S, Izumi Y, et al. A multicenter phase II prospective clinical trial of glucocorticoid for patients with untreated IgG4-related disease. Mod Rheumatol 2017;27:849-54. [Crossref] [PubMed]
- Maritati F, Peyronel F, Vaglio A. IgG4-related disease: a clinical perspective. Rheumatology (Oxford) 2020;59:iii123-31. [Crossref] [PubMed]


