
Real-time ultrasound-guided neuraxial anesthesia for cesarean section in parturients with previous internal fixation surgery for lumbar fracture: a case series
Introduction
Neuraxial anesthesia (i.e., spinal or epidural block) is the preferred type of anesthesia for cesarean section (CS) worldwide (1-3). Compared with general anesthesia (GA), neuraxial anesthesia may reduce the incidence of maternal airway complications and without potential adverse effects of general anesthetics on the newborn (4). Currently, GA is used almost exclusively in emergency CS or when neuraxial anesthesia has failed or is contraindicated (5). It remains unclear which anesthesia is most appropriate for CS in parturients who have undergone internal fixation surgery for lumbar fracture. Neuraxial anesthesia is not an absolute contraindication in these patients (6,7); however, postoperative distortion of the anatomy makes the block technically challenging and may increase the likelihood of failure, inadvertent dural puncture during epidural anesthesia, paresthesias, or unpredictable spread of the local anesthetic (8). Nevertheless, neuraxial anesthesia should be considered, as it offers undeniable advantages for CS. To date, there have been only a few case reports on the use of this technique in patients who have undergone internal fixation surgery for lumbar fracture (7,9).
In recent years, there has been an increasing interest in the use of ultrasound (US) for assisted or real-time guided neuraxial anesthesia, as it may help to increase the success rate. Preprocedural spinal US assessment can accurately determine the optimum best introduction site, angle, direction of approach, and depth to the epidural space, and subsequently reduce the number of attempts to administer neuraxial anesthesia (10-12). Real-time US guidance may offer the additional advantages of visualizing the needle tip and allowing adjustment of the trajectory (13-15).
Despite these potential advantages, there have been no reports on real-time US-guided neuraxial anesthesia for CS in parturients after internal fixation surgery for lumbar fracture. In this study, we report on our experience with 4 such patients.
Patients and methods
A retrospective case series of 4 patients with a history of internal fixation surgery for lumbar fracture who were scheduled for lower segment CS between March 2017 and March 2020 was conducted. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Parturient demographics and labor data
The demographics, labor data, previous surgical site, and anesthesia method are shown in Table 1.
Table 1
| Case No. | Age, years | Height, cm | Weight, kg | BMI, kg/m2 | Gestational age, weeks | Cesarean section indication | Previous surgical site | Anesthesia method |
|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 162 | 68.5 | 26.1 | 39 | Cephalopelvic disproportion | L1 to L3 | CSEA (L3–4) |
| 2 | 30 | 158 | 70.6 | 28.0 | 38 | Breech presentation | T12 to L2 | CSEA (L4–5) |
| 3 | 29 | 164 | 85.5 | 32.4 | 39 | Cephalopelvic disproportion | L3 to L4 | EA (L1–2) |
| 4 | 25 | 163 | 77.8 | 29.3 | 38 | Fetal macrosomia | T11 to L2 | CSEA (L4-5) |
BMI, body mass index; CSEA, combined spinal and epidural anesthesia; EA, epidural anesthesia.
History of lumbar fracture and choice of anesthesia
Case 1
The parturient had experienced an L2 vertebral compression fracture 2 years earlier, with no nerve injury complication. A previous radiograph showed bilateral pedicle screw-rod fixation from L1 to L3. The internal fixators were not removed preoperatively. For CS, combined spinal and epidural anesthesia (CSEA) was performed at the L3–4 interspace.
Case 2
Three years earlier, the parturient had undergone an internal fixation of a bilateral pedicle screw-rod from T12 to L2 caused by a L1 vertebral compression fracture without neurologic deficit. The internal fixators remained in her body. For CS, CSEA was performed at the L4–5 interspace.
Case 3
Seven years earlier, the parturient had presented with L3 and L4 open fractures accompanied by left L4 nerve root injury. Five years earlier, the lumbar internal fixators had been removed. Preoperatively, the parturient still had a neurological deficit of the left lower limb with L4 paresthesia and mild motor weakness (grade 4/5) of the left ankle dorsiflexion. The L1–2 interspace was chosen for epidural anesthesia for CS.
Case 4
The parturient had experienced T12 and L1 vertebral fractures without neurological injury 2 years earlier. Bilateral pedicle screws and rods had been fixed from T11 to L2 and were still in the body before delivery. For CS, CSEA was performed at the L4–5 interspace.
Preprocedural spinal US assessment
Routine monitoring (noninvasive blood pressure, pulse oximetry, and electrocardiography) and intravenous access were established after the parturients arrived in the operating room. The parturients were placed in the left lateral decubitus position with the lumbar spine flexed appropriately, which enabled simple control of the needle in a caudad-to-cephalad direction with the right hand for right-handed doctors, while the parturients’ right lateral decubitus position enabled left-handed doctors to control the needle. The lumbar spine was scanned using a curvilinear low-frequency (2–5 MHz) probe (Sonosite®, MicroMaxx, Bothwell, WA, USA; or NextGen LOGIQTM e, GE Healthcare, Milwaukee, WI, USA). The US image was optimized before the intervention by adjustment of parameters such as scanning depth, focus, and gain.
Previous plain radiographs of the spine showed no congenital vertebral anomaly, such as an L1 accessory rib or sacralization of the L5 vertebra, in any of the 4 parturients. Thus, the lumbar intervertebral space could be accurately identified using a known systematic US scanning protocol combining a counting-up approach from the L5-S1 junction with a counting-down approach from the T12 transverse process (identified by the presence of the 12th rib) in a paramedian sagittal plane (16,17).
The screw and rod fixation system can be easily identified as strong reflectors on US examination. They are shown as highlighted dashes bilaterally in the median transverse view (Figure 1) and a long, strong echogenic line in the paramedian sagittal view (Figure 2).
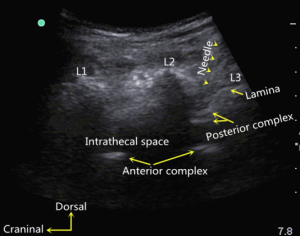
Considering the results of lumbar US scanning and previous spine radiographs, the choice of the target puncture site should not include the previous operation area. The main feature of scar tissue on US is an echogenic homogeneous or inhomogeneous irregular area, which may be surrounded by a hypoechoic halo (18). If excessive deep, permanent scar tissue is present, it is difficult to advance the needle. Therefore, we kept the needle away from the scar tissue when selecting a puncture site. The transducer was positioned 1–2 cm lateral to the midline spinous processes on the nondependent (up) side, and the lumbar spine was scanned in a paramedian sagittal oblique plane, as described by Chin et al. (17). The interlaminar space, in which the gap was relatively wide and the posterior complex and/or anterior complex could be visualized, was chosen as the target puncture site. The posterior complex includes the ligamentum flavum and posterior dura mater, which often appears as a single linear hyperechoic structure. Similarly, the anterior dura, posterior longitudinal ligament, and posterior aspect of the vertebral body or intervertebral disc can be visible as a single linear hyperechoic structure, that is the anterior complex (17,19). The target interlaminar space was maintained near the caudal edge (Figure 3) instead of the center of the US image to reduce the length of the puncture path and the probability of touching the lamina with the needle during the procedure. Then, the position of the transducer was marked on the skin.
Real-time US-guided neuraxial anesthesia
After sterilization of the lumbar skin, the US transducer was prepared by applying a thin layer of ultrasonic coupling agent onto the footprint and covering it with a sterile transducer sleeve. The US transducer was placed on the previously marked position. The intended needle insertion site was infiltrated with 2% lidocaine. An 18-gauge Tuohy needle was inserted from the caudal end of the probe and gradually advanced under real-time US guidance using an in-plane approach towards the target interlaminar space until the tip of the needle was seen to be approximately 1 cm away from the posterior complex. Then, the US probe was set aside to avoid an inadvertent dural puncture due to the single-handed needle insertion. The needle was advanced through the ligamentum flavum and into the epidural space using a loss-of-resistance (LOR) to saline technique for identification of the epidural space.
In parturients who were administered an epidural anesthetic for CS, an epidural catheter was inserted into the epidural space using a Tuohy needle, with 4 cm of the catheter remaining in the epidural space, and it was secured to the back. After aspiration of the catheter, 3 mL of 2% lidocaine with epinephrine (1:200,000) was administered as a test dose to exclude intravascular or intrathecal placement. The parturient was then returned to the supine position with a 15° left lateral tilt. A 0.75% solution of ropivacaine was injected through the epidural catheter at a rate of 5 mL every 5 minutes and titrated up to the T5 level of the block. When CSEA was planned for the CS, a 27-gauge pencil-point needle was advanced into the subarachnoid space through the Tuohy needle using a needle-through-needle technique. After confirmation of correct spinal needle placement using aspiration of cerebrospinal fluid, 3.0 mL of 0.5% ropivacaine (2.0 mL 0.75% ropivacaine + 1.0 mL cerebrospinal fluid) was injected. After withdrawal of the spinal needle, an epidural catheter was inserted into the epidural space. If the desired block level was not achieved after 10 minutes of subarachnoid block, rescue epidural injection of local anesthetic was performed.
Results
The internal fixators and deep scar tissue were well-identified using a preprocedural US scan, which was helpful in selecting the puncture site. The posterior and anterior complexes could be visualized in the 4 parturients. Paramedian lumbar epidural access was successfully performed in all parturients on the first attempt (single skin puncture with 1 or more needle passes) (15) by a single operator with real-time US guidance. The tip of the Tuohy needle was successfully advanced by a single operator with real-time US guidance in a paramedian sagittal oblique plane to be at a distance of approximately 1 cm from the posterior complex. Then, the tip of the needle was continued to advance into the epidural space using the LOR to saline technique.
The effect of neuraxial anesthesia was excellent in all parturients. There were no cases of anesthesia complications, such as back pain, postdural puncture headache, or new neurological deficits.
Discussion
General and neuraxial anesthesia are both safe for CS, but neuraxial anesthesia is still recommended as the gold standard anesthetic for most CS when balancing risks and benefits to the mother and her fetus (1,2,20). It is the preferred option due to lower fetal exposure to depressant drugs, lower risk of gastric content aspiration, difficulty of maternal intubation with GA, preservation of maternal consciousness during labor and feeling the joy of birth, and decreased requirement for postoperative analgesia (21,22). GA is currently used in only 5.8% of all cesarean deliveries (CDs) and 14.6% of emergent CDs in the US (23).
Published research suggests that neuraxial anesthesia is technically possible in most patients with a history of previous spinal surgery and may be recommended in obstetrics under certain conditions (24). Preexisting neurological deficits may be a specific concern for anesthesiologists. A retrospective study demonstrated that neuraxial anesthesia in patients with recent stable fractures of the spine was not associated with adverse neurological events (6). Lavelle et al. reported on 2 patients who received epidural anesthesia for CS after anterior spinal surgery (25). Majeed et al. and Yeo et al. reported that spinal anesthesia was successfully performed in parturients with scoliosis corrected with Harrington’s rod surgery (26,27). Most parturients may benefit more from neuraxial anesthesia than from GA (24), and therefore, we chose neuraxial anesthesia for CS in parturients after lumbar fracture operations.
Multiple prior case series have suggested that baseline neurologic symptoms of patients with pre-existing spinal canal pathology or nerve injury may worsen after neuraxial anesthesia or analgesia (28-30). However, neuraxial anesthesia can usually be administered safely in most patients with neurologic disease (31). The decision to administer neuraxial anesthetics in patients should be based on risk-to-benefit considerations. The third parturient in this study had baseline neurologic dysfunction and was obese, which presented difficulties to airway management. For this parturient, epidural anesthesia was safer for the mother and newborn than GA, despite the risk of worsening neurological symptoms.
Surgical scar tissue, which can form both extradurally and intradurally after spinal surgery, may block the diffusion of local anesthetic (32). Altered spinal anatomy due to previous surgery, presence of metal rods and screws, poor back flexion, scar tissue, and obliterated epidural space make neuraxial anesthesia technically difficult, and therefore, it frequently has a high failure rate and may lead to patchy or inadequate sensory block (33) and a high inadvertent dural puncture rate (34). Thus, the target puncture site of anesthesia should be chosen above or below the location of the previous internal fixation surgery (35), where the spinal column may be intact, and the lumbar puncture may be successful and safe. If the previous location of surgery is above the L3 level, CSEA is performed caudally. Otherwise, epidural anesthesia is chosen at the upper lumbar interspace.
Many studies have demonstrated that US is helpful in identifying the puncture site and measuring the puncture depth when performing neuraxial techniques, which increases the success rate, particularly in technically difficult cases (24,36-39). Preprocedural US scanning of the spine may reduce the risk of technical difficulties in patients with a previous history of lumbar surgery (26,27,40). The spinal pedicle screw and rod fixation system can be easily recognized as strong reflectors in the median transverse and paramedian sagittal views. It is advisable to circumvent the previous surgical area and deep scar tissue by using US scanning. The number of alternative lumbar interspaces to administer neuraxial anesthesia is reduced in patients with a history of lumbar surgery. Accurate advancement toward the target interspace can be performed under real-time US guidance, which may decrease the number of attempts and subsequent trauma (15).
Paramedian sagittal oblique sonograms of the ligamentum flavum and posterior dura are of superior quality to those obtained in the median transverse plane or median longitudinal plane (14,16,17,41). Thus, the target interspace was approached through a paramedian sagittal oblique plane under real-time US guidance, as described by Karmakar et al. (14). In the study by Karmakar et al., the target interspace was consistently maintained in the center of the US image (14). In practice, however, this approach makes the needle trajectory long, and the needle is easily blocked by the lamina because of the narrow laminar space. Needle contact with the vertebrae can lead to a failed epidural procedure and back pain (42). In our study, the target interspace was maintained near the caudal edge instead of the center of the US image, which reduced the length of the needle path and the contact between the needle and the lamina. The correlation between the position of the target interspace on US images and difficulty of the epidural procedure using real-time US guidance needs further study. Much of the needle tract is guided by real-time US, which allows the needle trajectory to be aimed at the target epidural space, and the remaining part is performed easily using the freehand method.
In conclusion, neuraxial anesthesia can be used carefully for CS in parturients who have undergone lumbar fracture operations. Real-time US guidance is helpful for the success of this type of anesthesia.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-223/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2016;124:270-300. [Crossref] [PubMed]
- Kim WH, Hur M, Park SK, Yoo S, Lim T, Yoon HK, Kim JT, Bahk JH. Comparison between general, spinal, epidural, and combined spinal-epidural anesthesia for cesarean delivery: a network meta-analysis. Int J Obstet Anesth 2019;37:5-15. [Crossref] [PubMed]
- Kinsella SM, Winton AL, Mushambi MC, Ramaswamy K, Swales H, Quinn AC, Popat M. Failed tracheal intubation during obstetric general anaesthesia: a literature review. Int J Obstet Anesth 2015;24:356-74. [Crossref] [PubMed]
- Rollins M, Lucero J. Overview of anesthetic considerations for Cesarean delivery. Br Med Bull 2012;101:105-25. [Crossref] [PubMed]
- Devroe S, Van de Velde M, Rex S. General anesthesia for caesarean section. Curr Opin Anaesthesiol 2015;28:240-6. [Crossref] [PubMed]
- Gurajala I, Iyengar R, Durga P, Gopinath R. Spinal and Epidural Anesthesia in Patients With Recent Stable Fractures of Vertebral Column. J Neurosurg Anesthesiol 2016;28:262-6. [Crossref] [PubMed]
- Rosaeg OP, Yarnell RW, Lindsay MP. The obstetrical anaesthesia assessment clinic: a review of six years experience. Can J Anaesth 1993;40:346-56. [Crossref] [PubMed]
- Walsh E, Zhang Y, Madden H, Lehrich J, Leffert L. Pragmatic approach to neuraxial anesthesia in obstetric patients with disorders of the vertebral column, spinal cord and neuromuscular system. Reg Anesth Pain Med 2021;46:258-67. [Crossref] [PubMed]
- Sharpe EE, Arendt KW, Jacob AK, Pasternak JJ. Anesthetic management of parturients with pre-existing paraplegia or tetraplegia: a case series. Int J Obstet Anesth 2015;24:77-84. [Crossref] [PubMed]
- Weiniger CF, Sharoni L. The use of ultrasound in obstetric anesthesia. Curr Opin Anaesthesiol 2017;30:306-12. [Crossref] [PubMed]
- Talati C, Arzola C, Carvalho JC. The Use of Ultrasonography in Obstetric Anesthesia. Anesthesiol Clin 2017;35:35-58. [Crossref] [PubMed]
- Chin A, Crooke B, Heywood L, Brijball R, Pelecanos AM, Abeypala W. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia 2018;73:466-73. [Crossref] [PubMed]
- Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol 2004;21:25-31. [PubMed]
- Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth 2009;102:845-54. [Crossref] [PubMed]
- Liu Y, Qian W, Ke XJ, Mei W. Real-time Ultrasound-guided Spinal Anesthesia Using a New Paramedian Transverse Approach. Curr Med Sci 2018;38:910-3. [Crossref] [PubMed]
- Tran D, Kamani AA, Al-Attas E, Lessoway VA, Massey S, Rohling RN. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth 2010;57:313-21. [Crossref] [PubMed]
- Chin KJ, Karmakar MK, Peng P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology 2011;114:1459-85. [Crossref] [PubMed]
- Drakonaki EE, Sudoł-Szopińska I, Sinopidis C, Givissis P. High resolution ultrasound for imaging complications of muscle injury: Is there an additional role for elastography? J Ultrason 2019;19:137-44. [Crossref] [PubMed]
- Elsharkawy H, Maheshwari A, Babazade R, Perlas A, Zaky S, Mounir-Soliman L. Real-time ultrasound-guided spinal anesthesia in patients with predicted difficult anatomy. Minerva Anestesiol 2017;83:465-73. [Crossref] [PubMed]
- Mancuso A, De Vivo A, Giacobbe A, Priola V, Maggio Savasta L, Guzzo M, De Vivo D, Mancuso A. General versus spinal anaesthesia for elective caesarean sections: effects on neonatal short-term outcome. A prospective randomised study. J Matern Fetal Neonatal Med 2010;23:1114-8. [Crossref] [PubMed]
- Ghaffari S, Dehghanpisheh L, Tavakkoli F, Mahmoudi H. The Effect of Spinal versus General Anesthesia on Quality of Life in Women Undergoing Cesarean Delivery on Maternal Request. Cureus 2018;10:e3715. [Crossref] [PubMed]
- Dağlı R, Dağlı SS. Anaesthetic Method Preference of Obstetricians for Caesarean Section. Turk J Anaesthesiol Reanim 2015;43:41-6. [Crossref] [PubMed]
- Juang J, Gabriel RA, Dutton RP, Palanisamy A, Urman RD. Choice of Anesthesia for Cesarean Delivery: An Analysis of the National Anesthesia Clinical Outcomes Registry. Anesth Analg 2017;124:1914-7. [Crossref] [PubMed]
- Vercauteren M, Waets P, Pitkänen M, Förster J. Neuraxial techniques in patients with pre-existing back impairment or prior spine interventions: a topical review with special reference to obstetrics. Acta Anaesthesiol Scand 2011;55:910-7. [Crossref] [PubMed]
- Lavelle WF, Demers E, Fuchs A, Carl AL. Pregnancy after anterior spinal surgery: fertility, cesarean-section rate, and the use of neuraxial anesthesia. Spine J 2009;9:271-4. [Crossref] [PubMed]
- Majeed A, Ahmed I, Alkahtani GJ, Altahtam NA. Ultrasound-guided continuous spinal anesthesia for cesarean section in a parturient with scoliosis corrected with Harrington's rod surgery. Saudi J Anaesth 2017;11:479-82. [Crossref] [PubMed]
- Yeo ST, French R. Combined spinal-epidural in the obstetric patient with Harrington rods assisted by ultrasonography. Br J Anaesth 1999;83:670-2. [Crossref] [PubMed]
- Hebl JR, Kopp SL, Schroeder DR, Horlocker TT. Neurologic complications after neuraxial anesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth Analg 2006;103:1294-9. [Crossref] [PubMed]
- Watson JC, Huntoon MA. Neurologic Evaluation and Management of Perioperative Nerve Injury. Reg Anesth Pain Med 2015;40:491-501. [Crossref] [PubMed]
- Kopp SL, Jacob AK, Hebl JR. Regional Anesthesia in Patients With Preexisting Neurologic Disease. Reg Anesth Pain Med 2015;40:467-78. [Crossref] [PubMed]
- Waters JFR. Neurologic Complications of Obstetric Anesthesia. Continuum (Minneap Minn) 2022;28:162-79. [Crossref] [PubMed]
- Sun KO. Spinal anaesthesia following previous spinal surgery. Eur J Anaesthesiol 1994;11:321-3. [PubMed]
- Kardash K, King BW, Datta S. Spinal anaesthesia for caesarean section after Harrington instrumentation. Can J Anaesth 1993;40:667-9. [Crossref] [PubMed]
- Crosby ET, Halpern SH. Obstetric epidural anaesthesia in patients with Harrington instrumentation. Can J Anaesth 1989;36:693-6. [Crossref] [PubMed]
- Ghaly RF, Tverdohleb T, Candido KD, Knezevic NN. Lumbar epidural analgesia for labor in a parturient with a history of surgery for lumbar intradural ependymoma: Literature review and case presentation. Surg Neurol Int 2018;9:211. [Crossref] [PubMed]
- Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth 2016;28:12-6. [Crossref] [PubMed]
- Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth 2014;28:413-9. [Crossref] [PubMed]
- Ekinci M, Alici HA, Ahiskalioglu A, Ince I, Aksoy M, Celik EC, Dostbil A, Celik M, Baysal PK, Golboyu BE, Yeksan AN. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth 2017;37:82-5. [Crossref] [PubMed]
- Chin KJ, Perlas A. Ultrasonography of the lumbar spine for neuraxial and lumbar plexus blocks. Curr Opin Anaesthesiol 2011;24:567-72. [Crossref] [PubMed]
- Chin KJ, Macfarlane AJ, Chan V, Brull R. The use of ultrasound to facilitate spinal anesthesia in a patient with previous lumbar laminectomy and fusion: a case report. J Clin Ultrasound 2009;37:482-5. [Crossref] [PubMed]
- Grau T, Leipold RW, Horter J, Conradi R, Martin EO, Motsch J. Paramedian access to the epidural space: the optimum window for ultrasound imaging. J Clin Anesth 2001;13:213-7. [Crossref] [PubMed]
- Chen GS, Chang YC, Chang Y, Cheng JS. A prototype axial ultrasound needle guide to reduce epidural bone contact. Anaesthesia 2014;69:746-51. [Crossref] [PubMed]




