
Venous collaterals in acute ischemic stroke patients after endovascular treatments: a novel scoring system using 4D computed tomography angiography
Introduction
Vascular neuroimaging plays a decisive role in the determination of the optimal therapeutic method for patients with acute ischemic stroke (AIS). A recently published meta-analysis found that arterial cerebral collaterals were significantly associated with clinical and safety outcomes, albeit with a prognostic accuracy range of 48% to 66% (1). The effect of the venous collaterals, compared to that of the arterial collaterals, in patients with AIS has not been thoroughly elucidated (2,3). The venous system has traditionally been considered to play a secondary role in the microcirculation of ischemic brain tissues, but the venous system may actually have crucial effects on cerebral blood flow under both normal and pathological conditions. The cerebral venous system comprises about 70% of the total cerebral blood volume (CBV) and plays a pivotal role in oxygen exchange (4). Physiologically, the alternation of venous drainage can indicate a change of cerebral perfusion and the optimization of the oxygen uptake mechanism (5). Available evidence suggests that there may be a putative link between venous collaterals and long-term clinical outcome in patients with AIS who have undergone reperfusion therapy (4,6-11). The venous status may reflect the comprehensive state of blood flow compensation and tissue perfusion through the collateral branch of the microcirculation. Although previous research has emphasized the potential supplementary role of venous collaterals during the clinical decision-making process of AIS, the following problems remain: (I) the commonly used scoring methods for evaluating venous collateral circulation, such as prognostic evaluation based on cortical vein score difference in stroke score (PRECISE) (12) and cortical vein opacification score (COVES) (13), are mainly based on single-phase computed tomography angiography (sCTA), which may lose some vascular information and result in underestimation; (II) the published venous collateral studies based on four-dimensional computed tomography angiography (4D CTA) have mostly focused on the analysis of regional abnormal features of venous drainage and have not proposed a comprehensive venous collateral scoring method; and (III) most studies have targeted patients with intravenous thrombolysis. In the context of the extensive application of endovascular treatments (EVTs) for AIS, it is necessary to evaluate the correlation between venous collateral status and clinical outcome in AIS patients who have undergone EVTs.
This study aimed to establish a novel cortical venous collateral score based on 4D CTA (4D-VCS) to comprehensively assess the status of cortical venous collateral circulation in patients with AIS, and to evaluate the predictive efficacy of this method for the clinical outcome of AIS patients after EVTs. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-245/rc).
Methods
Study population
This was a retrospective case-control study. The data of all patients with AIS in the emergency department of Beijing Hospital who received EVTs from March 2016 to August 2021 were analyzed, including patients who met the criteria for intravenous thrombolysis and received recombinant tissue plasminogen activator (rtPA) treatment before EVTs (Figure 1).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Beijing Hospital approved the study, and individual consent for this retrospective analysis was waived.
The inclusion criteria were as follows: (I) age ≥18 years old; (II) patients underwent EVTs within 24 hours after symptom onset; and (III) one-stop 4D CTA-CT perfusion (CTP) examination revealed large vessel occlusion of the unilateral anterior circulation. The exclusion criteria were as follows: (I) non-contrast CT scan (NCCT) revealed intracranial hemorrhage or subarachnoid hemorrhage; (II) previous large cerebral infarction of the ipsilateral cerebral hemisphere (infarct size equal to or larger than two-thirds of the middle cerebral artery territory); (III) moderate/severe stenosis and/or occlusion of the contralateral arteries; and (IV) incomplete clinical, laboratory, or imaging data.
Imaging protocols
All patients underwent NCCT to exclude intracranial hemorrhage (scan parameters: 80 kV/200 mAs/detector and 0.5×80/volume scan). Then, one-stop whole-brain dynamic volume 4D CTA-CTP examinations (Aquilion ONE, Canon Medical Systems, Otawara, Japan) were performed using a 320×0.5 mm detector row CT. Non-ionic iodinated contrast medium (0.6 mL/kg; Iopamidol, Braccosine, Shanghai, China) was injected intravenously followed by 30 mL saline with a 2-channel high-pressure injector. A dynamic volume perfusion scan was performed (scan parameters: 80 kV, 100 mAs, coverage area of 160 mm, layer thickness of 0.5 mm) 7 s after contrast injection.
Venous collateral scoring methods
All CTA images were anonymized and reviewed independently at the same workstation by two experienced neuroradiologists who were blinded to the cases’ clinical information and follow-up imaging information. The vascular images of each patient were evaluated for venous collaterals according to the following 3 criteria.
PRECISE (12)
The veins included for the assessment were the superficial anastomotic veins [superficial middle cerebral vein (SMCV), vein of Trolard (VOT), and vein of Labbé (VOL)] and the basal vein of Rosenthal (BVR). The veins were scored based on sCTA (the phase in which the artery had peak CT value was selected as the arterial phase from the 19 phases to generate images of sCTA for each patient). A score of 0, 1, or 2 was given for absent, partial, or full constitution, respectively. “Absent” was defined as a vascular contrast density lower than 100 HU; “Partial” was defined as a contrast density higher than 100 HU, but lower than that of the contralateral hemisphere; “Full” was defined as a contrast density comparable to the corresponding vein in the contralateral hemisphere on visual analysis.
COVES (13)
The VOL, sphenoparietal sinus, and SMCV were quantified on sCTA as follows: 0, not visible; 1, moderate opacification; and 2, full opacification.
4D-VCS
The veins included for evaluation were SMCV, sphenoparietal sinus, VOT, and VOL. These veins were chosen because they account for almost all venous drainage in the middle cerebral artery territory and show limited anatomical variability when compared to other cortical veins. Each vein was graded on a scale from 0 to 4 along the time shaft: a score of 0 indicated absent collateral vessels in the ischemic area in any phase; a score of 1 indicated partial collateral vessels were revealed until the late venous phase; a score of 2 indicated partial collateral vessels in the venous phase, and no changes until the late venous phase; a score of 3 indicated full venous vessels until the late venous phase (regardless of whether partial collateral vessels were revealed in the venous phase or not); and a score of 4 indicated full venous vessels in the venous phase. The 4D-VCS ranged from 0 (no opacification of the 4 venous pathways) to 16 (full opacification of the 4 venous pathways). “Absent” was defined as a vascular contrast density lower than 100 HU. “Full” was defined as a contrast density comparable to that of the corresponding vein in the contralateral hemisphere on visual analysis. “Partial” was defined as a contrast density higher than 100 HU but lower than that of the contralateral hemisphere. According to the time density curve (TDC), the venous phase was defined as the peak of the venous curve and the late venous phase was defined as the phase after the peak of the venous curve (Figures 2,3).
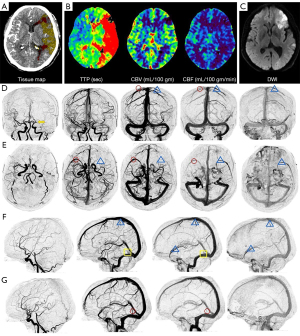

Other imaging parameters analysis
For the arterial collateral vessels, we used the modified collateral circulation scoring system on 4D CTA with a range of 0 to 4 (14): 0 points indicated no or few collateral vessels (<50% flow of the normal side) in the ischemic area in any phase; 1 point indicated partial arterial collaterals (between 50% and 100% flow of the normal side) until the late venous phase; 2 points indicated partial arterial collaterals (between 50% and 100% flow of the normal side) before the venous phase; 3 points indicated complete arterial collaterals (≥100% flow of the normal side) in the late venous phase (regardless of whether partial collateral vessels were found before the venous phase or not); and 4 points indicated that complete collateral circulation (≥100% flow of the normal side) was found before venous phase.
The dynamic progression of the internal cerebral vein (ICV) was categorized into 3 types (11): type 1 ICV progression was defined as ipsilateral ICV showing less opacification than the asymptomatic side; type 2 was defined as ipsilateral ICV showing equal opacification to the asymptomatic side; and type 3 was defined as ipsilateral ICV showing more opacification than the asymptomatic side.
The Alberta stroke program early CT score (ASPECTS; a 10-point scale grading system) was used to evaluate early ischemic changes of the brain parenchyma on NCCT (15).
Vitrea (Vital Images, Minnetonka, MN, USA) was used to perform all the CTP analyses. The TDCs for the input artery and output vein were obtained from the internal carotid artery in the normal side and superior sagittal sinus, respectively. A 38% reduction in CBV with a 5.3-s increase of time to peak (TTP) indicated infarct core (IC), and a 5.3-s increase of TTP without CBV reduction indicated ischemic penumbra (IP). The mismatch ratio (MMR) was the IP volume divided by the IC volume (16).
Clot burden score (CBS) was scored based on maximum intensity projection, which has been described by Wei et al. (17).
Clinical data collection
The following information was collected: (I) the National Institutes of Health Stroke Scale (NIHSS; scale ranged from 0 to 42 points); (II) general data, including age and gender; (III) the risk factors of cerebrovascular disease; and (IV) the type of stroke, which was determined based on Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification (18).
Outcomes
Recanalization of the occluded artery was evaluated on the final digital subtraction angiography and classified according to the modified Thrombolysis In Cerebral Ischemia (mTICI) score. Successful recanalization was defined as mTICI grade ≥2b. The modified Rankin Scale (mRS) was used to evaluate the patients’ clinical outcome after 3 months. The patients were regarded as having a good clinical outcome if the mRS score was <2 and a poor clinical outcome if the mRS score was ≥3 (19). Final infarct volume (FIV) was defined on NCCT or magnetic resonance imaging (MRI) after 2–7 days.
Statistical analysis
Continuous variables were described as median (interquartile range) for skewed data, and categorical data were represented as a frequency distribution. Mann-Whitney U, Pearson’s chi-square, and Fisher’s exact tests were used to compare the baseline information between the good and poor outcome groups. All patients were assessed twice by each observer independently. Interobserver agreement was described by the intraclass correlation coefficient (ICC). Spearman’s correlation analysis was used to analyze the correlation of different venous collateral scoring systems with FIV, mRS score, and artery collateral score. Receiver operating characteristic (ROC) curves of different venous collateral scoring systems were used to analyze the sensitivity and specificity of the outcome evaluation. Multivariate logistic regression analysis of baseline clinical characteristics and collateral scores was used to identify each model’s prognostic value. A total of 6 models were developed: (I) clinical information [age, NIHSS, thrombus location, atrial fibrillation (AF), coronary heart disease (CHD), IC volume, CBS, ICV type, and MMR]; (II) clinical information and arterial collateral score; (III) clinical information and 4D-VCS; (IV) clinical information and PRECISE; (V) clinical information and COVES; and (VI) clinical information, 4D-VCS, and arterial collateral score. Model 1 was compared with models 3, 4, 5, and 6, and model 2 was compared with models 3, 4, 5, and 6. The DeLong test was used to test differences in the area under the curve (AUC) values of the ROC curve. We calculated the adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of different variables in relation to poor outcomes. The ability of 4D-VCS to identify clinical outcome was validated internally using 1,000 bootstrap replications of the ROC. Results were considered significant for 2-tailed P<0.05. Statistical analysis was performed using the software SPSS 25.0 (SPSS, IBM Corp., Armonk, NY, USA) and MedCalc 19.6 (MedCalc Software, Mariakerke, Belgium).
Results
Study population
In total, 107 patients were consecutively enrolled in this study, including 55 males (51.4%) and 52 females (48.6%). Significant differences between the good outcome group and the poor outcome group were revealed in many variables including age, NIHSS, thrombus location, IC volume, MMR, FIV, arterial collateral score, CBS, different venous collateral scores, and ICV type (all P<0.05). Detailed information is summarized in Table 1.
Table 1
| Characteristics | All patients (n=107) | Good outcome (mRS 0–2) (n=60) | Poor outcome (mRS 3–6) (n=47) | P value |
|---|---|---|---|---|
| Age, years, median (IQR) | 73.00 (62.00, 83.00) | 68.00 (63.25, 79.50) | 80.00 (68.00, 85.00) | 0.003** |
| Women, n (%) | 52 (48.60) | 24 (40.0) | 28 (59.6) | 0.053 |
| NIHSS, median (IQR) | 13.00 (8.00, 17.00) | 9.50 (6.00, 14.75) | 15.00 (13.00, 21.00) | <0.001*** |
| Stroke etiology, n (%) | 0.409 | |||
| Cardioembolism | 58 (54.2) | 30 (50.0) | 28 (59.6) | |
| Large-artery atherosclerosis | 37 (34.6) | 24 (40.0) | 13 (27.7) | |
| Other determined | 1 (0.9) | 0 (0) | 1 (2.1) | |
| Cryptogenic | 11 (10.3) | 6 (10.0) | 5 (10.6) | |
| Thrombus location, n (%) | 0.025* | |||
| Tandem | 8 (7.5) | 5 (8.3) | 3 (6.4) | |
| ICA | 39 (36.4) | 17 (28.3) | 22 (46.8) | |
| Segment M1 | 41 (38.3) | 22 (36.7) | 19 (40.4) | |
| Segment M2 | 19 (17.8) | 16 (26.7) | 3 (6.4) | |
| Preoperative IVT, n (%) | 21 (19.60) | 12 (20.0) | 9 (19.1) | 1.000 |
| mTICI, n (%) | 0.056 | |||
| 0–2a | 11 (10.3) | 3 (5.0) | 8 (17.0) | |
| 2b–3 | 96 (89.7) | 57 (95.0) | 39 (83.0) | |
| Risk factors, n (%) | ||||
| Smoking | 34 (31.8) | 29 (31.9) | 4 (36.4) | 0.059 |
| AF | 44 (41.1) | 18 (30.0) | 26 (55.3) | 0.010 |
| Hypertension | 73 (68.2) | 41(68.3) | 32 (68.1) | 1.000 |
| Diabetes mellitus | 33 (30.8) | 17 (28.3) | 16 (34.0) | 0.535 |
| Hyperlipidemia | 56 (52.3) | 30 (50.0) | 26 (55.3) | 0.697 |
| CHD | 40 (37.4) | 17 (28.3) | 23 (48.9) | 0.044 |
| Previous stroke | 45 (42.1) | 25 (41.7) | 20 (42.6) | 1.000 |
| Current anticoagulant use | 13 (12.1) | 7 (11.7) | 6 (12.8) | 1.000 |
| Current antiplatelet use | 37 (34.6) | 21 (35.0) | 16 (34.0) | 1.000 |
| Imaging examination | ||||
| IC volume (mL), median (IQR) | 34.34 (15.20, 69.37) | 21.74 (6.14, 38.51) | 69.37 (41.20, 122.83) | <0.001*** |
| IP volume (mL), median (IQR) | 90.15 (51.10, 141.88) | 88.51 (45.89, 131.35) | 90.20 (65.29, 142.76) | 0.183 |
| MMR, median (IQR) | 2.51 (1.23, 5.40) | 4.40 (2.26, 7.55) | 1.14 (0.71, 2.67) | <0.001*** |
| FIV (mL), median (IQR) | 43.39 (12.51, 120.75) | 27.63 (7.36, 46.21) | 108.15 (55.04, 247.67) | <0.001*** |
| ASPECTS, median (IQR) | 7.00 (5.00, 8.00) | 7.00 (6.00, 8.00) | 7.00 (3.00, 8.00) | 0.134 |
| Artery collateral scores, median (IQR) | 3.00 (1.00, 3.00) | 3.00 (3.00, 4.00) | 1.00 (1.00, 2.00) | <0.001*** |
| CBS, median (IQR) | 6.00 (1.00, 8.00) | 6.00 (3.25, 9.00) | 3.00 (0, 6.00) | 0.002** |
| Total PRECISE scores, median (IQR) | 3.00 (2.00, 4.00) | 4.00 (2.00, 6.00) | 2.00 (1.00, 3.00) | <0.001*** |
| SMCV, median (IQR) | 0 (0, 1.00) | 0.50 (0, 2.00) | 0 (0, 0) | <0.001*** |
| VOL, median (IQR) | 1.00 (0, 1.00) | 1.00 (0, 2.00) | 0 (0, 0) | <0.001*** |
| VOT, median (IQR) | 1.00 (0, 1.00) | 1.00 (1.00, 2.00) | 1.00 (0, 1.00) | <0.001*** |
| BVR, median (IQR) | 2.00 (0, 2.00) | 2.00 (0, 2.00) | 1.00 (0, 2.00) | 0.008** |
| Total COVES scores | 1.00 (0.00, 3.00) | 2.00 (1.00, 4.00) | 0 (0, 1.00) | <0.001*** |
| SMCV, median (IQR) | 0 (0, 1.00) | 0 (0, 2.00) | 0 (0, 0) | <0.001*** |
| Sphenoid sinus, median (IQR) | 0 (0, 1.00) | 0 (0, 2.00) | 0 (0, 0) | 0.003** |
| VOL, median (IQR) | 0 (0, 1.00) | 1.00 (0, 2.00) | 0 (0, 1.00) | <0.001*** |
| Total 4D-VCS, median (IQR) | 9.00 (5.00, 11.00) | 10.00 (9.00, 13.00) | 5.00 (3.00, 7.00) | <0.001*** |
| SMCV, median (IQR) | 2.00 (0, 3.00) | 3.00 (2.00, 4.00) | 0 (0, 1.00) | <0.001*** |
| Sphenoid sinus, median (IQR) | 2.00 (0, 3.00) | 3.00 (1.00, 4.00) | 0 (0, 1.00) | <0.001*** |
| VOL, median (IQR) | 2.00 (0, 3.00) | 3.00 (1.00, 4.00) | 1.00 (0, 2.00) | <0.001*** |
| VOT, median (IQR) | 3.00 (1.00, 3.00) | 3.00 (3.00, 4.00) | 1.00 (1.00, 3.00) | <0.001*** |
| ICV types, n (%) | 0.002** | |||
| Type 1 | 50 (46.7) | 20 (33.3) | 30 (63.8) | |
| Type 2 | 55 (51.4) | 39 (65.0) | 16 (34.0) | |
| Type 3 | 2 (1.9) | 1 (1.7) | 1 (2.1) |
*, P<0.05; **, P<0.01; ***, P<0.001. mRS, modified Rankin Scale; IQR, interquartile range; NIHSS, National Institute of Health Stroke Scale; ICA, internal carotid artery; IVT, intravenous thrombolysis; mTICI, modified thrombolysis in cerebral infarction; AF, atrial fibrillation; CHD, coronary heart disease; IC, ischemic core; IP, ischemic penumbra; MMR, mismatch ratio; FIV, final infarct volume; ASPECTS, Alberta stroke program early CT score; CBS, clot burden score; SMCV, superficial middle cerebral vein; VOL, the vein of Labbé; VOT, the vein of Trolard; BVR, basal vein of Rosenthal; ICV, internal cerebral vein asymmetry; PRECISE, prognostic evaluation based on cortical vein score difference in stroke score; COVES, cortical vein opacification score; 4D-VCS, venous collateral score based on 4D CTA.
Relationship of different venous collateral scores with FIV, mRS score, and arterial collateral score
PRECISE (r=−0.462; 95% CI: −0.603 to −0.312; P<0.001), COVES (r=−0.379; 95% CI: −0.532 to −0.215; P<0.001), and 4D-VCS score (r=−0.615; 95% CI: −0.737 to −0.473; P<0.001) showed negative correlations with FIV. PRECISE (r=−0.530; 95% CI: −0.672 to −0.382; P<0.001), COVES (r=−0.432; 95% CI: −0.600 to −0.281; P<0.001), and 4D-VCS (r=−0.706; 95% CI: −0.789 to −0.602; P<0.001) showed significant negative correlations with mRS score. PRECISE (r=0.575; 95% CI: 0.434 to 0.702; P<0.001), COVES (r=0.555; 95% CI: 0.433 to 0.683; P<0.001), and 4D-VCS (r=0.769; 95% CI: 0.678 to 0.838; P<0.001) all showed moderate negative correlations with arterial collateral score (Table 2).
Table 2
| Score | FIV | mRS scores | Artery collateral scores | |||||
|---|---|---|---|---|---|---|---|---|
| r | 95% CI | r | 95% CI | r | 95% CI | |||
| PRECISE | −0.462*** | −0.603, −0.312 | −0.530*** | −0.672, −0.382 | 0.575*** | 0.434, 0.702 | ||
| COVES | −0.379*** | −0.532, −0.215 | −0.432*** | −0.600, −0.281 | 0.555*** | 0.433, 0.683 | ||
| 4D-VCS | −0.615*** | −0.737, −0.473 | −0.706*** | −0.789, −0.602 | 0.769*** | 0.678, 0.838 | ||
***, P<0.001. FIV, final infarct volume; mRS, modified Rankin Scale; PRECISE, prognostic evaluation based on cortical vein score difference in stroke score; COVES, cortical vein opacification score; 4D-VCS, venous collateral score based on 4D CTA; CI, confidence interval.
Association of collateral circulation and clinical outcome
The results are shown in Figure 4: 4D-VCS had the best correlation with outcome. The AUC of 4D-VCS was 0.92 (95% CI: 0.85 to 0.96; P<0.0001). The optimal cutoff value identified by the Youden approach was 7, with a sensitivity of 0.85 and a specificity of 0.87. The AUCs were 0.80 (95% CI: 0.71 to 0.87) for PRECISE, 0.75 (95% CI: 0.66 to 0.83) for COVES, and 0.90 (95% CI: 0.83 to 0.95) for arterial collateral score.
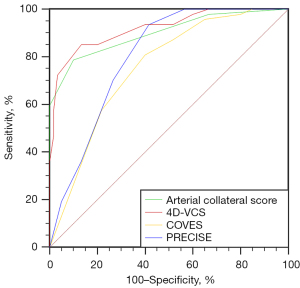
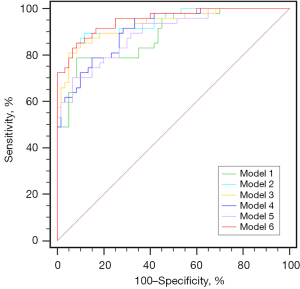
Table 3 summarizes the results of the 6 multivariable models. In model 1, NIHSS (OR =1.14; 95% CI: 1.02 to 1.27; P<0.05) and IC volume (OR =1.04; 95% CI: 1.02 to 1.07; P<0.05) were independent predictive factors of clinical outcome. The arterial collateral score (OR =0.14; 95% CI: 0.05 to 0.37; P<0.05) and 4D-VCS (OR =0.56; 95% CI: 0.41 to 0.78; P<0.05) were independent predictive factors of prognosis in model 2 and model 3, respectively. NIHSS (OR =1.14; 95% CI: 1.01 to 1.30; P<0.05), IC volume (OR =1.03; 95% CI: 1.01 to 1.06; P<0.05), and PRECISE (OR =0.63; 95% CI: 0.45 to 0.89; P<0.05) were independent predictors of clinical outcome in model 4. NIHSS (OR =1.14; 95% CI: 1.02 to 1.29; P<0.05) and IC volume (OR =1.04; 95% CI: 1.01 to 1.07; P<0.05) were independent predictors of clinical outcome in model 5. The arterial collateral score (OR =0.22; 95% CI: 0.07 to 0.67; P<0.05) was an independent predictive factor of prognosis in model 6. The ROC curve analysis (Figure 5) showed the AUC of all 6 models. Compared with model 1, there was a statistically significant difference between model 1 (AUC, 0.89; 95% CI: 0.81 to 0.94) and model 2 (AUC, 0.94; 95% CI: 0.88 to 0.98) (P=0.025), between model 1 (AUC, 0.89; 95% CI: 0.81 to 0.94) and model 3 (AUC, 0.93; 95% CI: 0.87 to 0.97) (P=0.045), and also between model 1 (AUC, 0.89; 95% CI: 0.81 to 0.94) and model 6 (AUC, 0.95; 95% CI: 0.89 to 0.98) (P=0.011). Compared with model 2, there was a statistically significant difference between model 2 (AUC, 0.94; 95% CI: 0.88 to 0.98) and model 5 (AUC, 0.89; 95% CI: 0.82 to 0.94) (P=0.032).
Table 3
| Predictors | OR with 95% CI | |||||
|---|---|---|---|---|---|---|
| Model 1 (clinical information) | Model 2 (clinical information + arterial collateral scores) | Model 3 (clinical information + 4D-VCS) | Model 4 (clinical information + PRECISE) | Model 5 (clinical information + COVES) | Model 6 (clinical information + 4D-VCS + arterial collateral scores) | |
| Age | 1.03 (0.98–1.08) | 1.05 (0.99–1.13) | 1.03 (0.97–1.09) | 1.02 (0.97–1.08) | 1.03 (0.98–1.08) | 1.04 (0.98–1.12) |
| NIHSS | 1.14* (1.02–1.27) | 1.10 (0.96–1.26) | 1.08 (0.94–1.24) | 1.14* (1.01–1.30) | 1.14* (1.02–1.29) | 1.08 (0.93–1.25) |
| Thrombus location | 0.95 (0.38–2.38) | 0.70 (0.20–2.46) | 1.03 (0.34–3.12) | 0.98 (0.36–2.68) | 0.93 (0.35–2.46) | 0.83 (0.23–2.99) |
| AF | 1.51 (0.46–4.95) | 1.39 (0.29–6.75) | 0.71 (0.15–3.25) | 1.50 (0.42–5.32) | 1.34 (0.40–4.52) | 0.93 (0.16–5.28) |
| CHD | 0.67 (0.20–2.21) | 0.45 (0.10–2.14) | 1.00 (0.24–4.10) | 0.66 (0.18–2.42) | 0.74 (0.21–2.58) | 0.59 (0.12–3.01) |
| IC volume | 1.04* (1.02–1.07) | 1.03 (0.99–1.07) | 1.03 (0.99–1.06) | 1.03* (1.01–1.06) | 1.04* (1.01–1.07) | 1.03 (0.98–1.07) |
| MMR | 1.02 (0.91–1.15) | 1.04 (0.91–1.18) | 1.04 (0.92–1.18) | 1.01 (0.88–1.15) | 1.00 (0.88–1.14) | 1.04 (0.91–1.12) |
| CBS | 1.02 (0.81–1.30) | 1.18 (0.81–1.71) | 1.11 (0.82–1.51) | 1.02 (0.79–1.31) | 1.08 (0.83–1.39) | 1.17 (0.80–1.70) |
| ICV types | 0.71 (0.24–2.12) | 0.41 (0.09–1.87) | 0.60 (0.14–2.65) | 0.83 (0.26–2.66) | 0.82 (0.26–2.55) | 0.45 (0.09–2.35) |
| Artery collateral scores | – | 0.14* (0.05–0.37) | – | – | – | 0.22* (0.07–0.67) |
| 4D-VCS | – | – | 0.56* (0.41–0.78) | – | – | 0.77 (0.54–1.08) |
| PRECISE | – | – | – | 0.63* (0.45–0.89) | – | – |
| COVES | – | – | – | – | 0.70 (0.49–1.01) | – |
| Model statistics | ||||||
| AUC (95% CI) | 0.89* (0.81–0.94) | 0.94* (0.88–0.98) | 0.93* (0.87–0.97) | 0.91* (0.84–0.96) | 0.89* (0.82–0.94) | 0.95* (0.89–0.98) |
| Sensitivity (%) | 78.72 | 89.36 | 80.85 | 70.21 | 78.72 | 85.11 |
| Specificity (%) | 91.67 | 88.33 | 95.00 | 93.33 | 85.00 | 91.67 |
| DeLong test P value compared with model 1 | – | 0.025* | 0.045* | 0.151 | 0.615 | 0.011* |
| DeLong test P value compared with model 2 | 0.025* | – | 0.618 | 0.113 | 0.032* | 0.352 |
*P<0.05 as the threshold for statistical significance. NIHSS, National Institute of Health Stroke Scale; AF, atrial fibrillation; CHD, coronary heart disease; MMR, mismatch ratio; CBS, clot burden score; IC, ischemic core; ICV, internal cerebral vein asymmetry; PRECISE, prognostic evaluation based on cortical vein score difference in stroke score; COVES, cortical vein opacification score; 4D-VCS, venous collateral score based on 4D CTA; 4D CTA, 4-dimensional computed tomography angiography; AUC, area under the curve; CI, confidence interval; OR, odds ratio.
Interobserver analysis and validation of the prognostic model
The ICC among the 2 observers was 0.89 on arterial collateral score, 0.90 on PRECISE, 0.94 on COVES, and 0.89 on 4D-VCS.
The bootstrap method was used to further validate the stability of the prognostic model, and the AUC was 0.921, which suggested that no overfitting had occurred.
Discussion
Although the advancement of EVTs and imaging technology has remarkably increased the successful recanalization rate in patients with AIS, the rate of good outcomes (3-month mRS <2) is only 33–71%, indicating that a considerable proportion of patients with AIS still experience poor clinical outcomes (20-22). The outcome of AIS treatment may be related not only to arterial vessels but also to venous vessels. Although methods already exist to score venous collateral circulation using sCTA, this study intended to establish an improved venous collateral circulation scoring system using 4D CTA to better evaluate the venous collateral vessels. Through a retrospective analysis of the data of 107 patients with AIS who received EVTs in a single center, we found that the status of venous collaterals based on 4D CTA exhibited effective prediction of the 3-month clinical outcome. This was not inferior to the prediction effect of the arterial collaterals, which suggested that the superposition of 4D-VCS and clinical factors could improve the predictive accuracy of outcomes of AIS patients compared with other venous scoring methods.
Existing evidence has shown that the following alterations may occur in the venous vessels in the hypoperfusion area of AIS: morphological changes of the venous lumen due to contraction or passive compression, the obstruction of venules resulting from microthrombotic aggregation, and the formation of new microvascular structures. All of the abovementioned changes result in imaging findings such as poor development and delayed development of the drainage veins in the ischemic area (23,24). Parthasarathy et al. used the PRECISE score based on sCTA to evaluate venous status and found that cortical vein drainage, not the deep venous drainage patterns on sCTA, can predict the prognosis of patients with anterior circulation large vessel occlusion (5). Parthasarathy et al. later proposed a combined arterial and venous grading scale (CRISP) to assess the 4 superficial drainage veins (SMCV, VOL, VOT, and BVR) on the affected side, as well as the compensatory factors of the arterial collaterals. This scoring system predicted the clinical outcomes more accurately in patients with acute large vessel occlusion of the anterior circulation (12). Zhang et al. investigated the development of 3 superficial drainage veins (SMCV, VOL, and VOT) after acute stroke using 3D-CTV and 4D CTA and found that the non-opacification of SMCV was strongly related to serious insufficient cerebral perfusion and poor clinical outcomes after intravenous thrombolysis (6). Bhaskar et al. observed that AIS patients with larger vessel occlusion were more prone to the phenomenon of delayed cortical vein filling in the late vein stage, which was highly correlated with 24-hour poor reperfusion status after intravenous thrombolysis (7). Although emerging studies have emphasized the potential complementary role of the venous collaterals in the clinical decision-making process, they have had some limitations. Most of such studies were mainly based on sCTA, which ignores the late filling cortical veins. In addition, previous studies have mostly focused on the analysis of the clinical outcome of intravenous thrombolysis. Therefore, the present study aimed to establish an improved venous collateral scoring system based on 4D CTA to evaluate the venous collateral circulation of patients with AIS who have undergone EVTs.
The 4D-VCS with a scale of 0–16 points based on 4D CTA was established to evaluate the venous collateral vessels along the time shaft and determine the velocity of collateral blood flow at the venous phase and late venous phase. This method can not only improve the accuracy of diagnosis, but can also make the diagnostic results more reliable and repeatable. The equipment used in this study was a 320-row dynamic volume CT. The whole-brain information can be collected within 60 s by 1 contrast injection, and the radiation dose of the examination is about 4–6 mSv (25).
The results of this study indicated that 4D-VCS was an independent factor affecting clinical outcome. We established different models by combining clinical factors and different venous collateral circulation scoring methods. The 4D-VCS method exhibited the best prediction value among the 3 venous collateral circulation scoring methods and was not inferior to the arterial collateral score in predicting clinical outcome (AUC =0.94 vs. AUC =0.93; P=0.618). However, in model 6, 4D-VCS was not an independent predictor. We interpret the possible reasons for this as follows: Firstly, the small sample size may have contributed to this result. Secondly, severe multicollinearity between venous the collateral circulation and the arterial collateral circulation could be one of the reasons why 4D-VCS was not statistically significant in model 6. However, the general trend can be seen in that the predictive value of model 6 was increased after adding 4D-VCS (AUC, 0.95; 95% CI: 0.89 to 0.98). Interobserver analysis and internal verification validated the high stability of 4D-VCS. The AUC of 4D-VCS used for clinical outcome prediction was 0.92. The cutoff value suggested that patients with a 4D-VCS of 7 and below were more likely to have poor outcomes 90 days after EVTs, and patients with a score higher than 7 were more likely to obtain functional independence. Therefore, our findings may provide a simple reference for predicting clinical outcomes. The venous collateral status was negatively correlated with poor outcome rate and FIV but positively correlated with the compensatory status of the arterial collateral vessels. These results suggested that the venous collateral circulation could be used as an additional imaging marker for patients with AIS. Careful observation of the venous collateral status on the affected side is critical for the prediction of clinical outcome.
This study had some limitations. Firstly, the sample size was relatively small; therefore, it is necessary to expand the sample size and conduct multicenter registration research to verify the results. Secondly, there was a certain proportion of congenital cortical vein loss in the included patients. The 4 cortical veins chosen for the 4D-VCS in this study had less anatomic variability than that of other veins, and this could reduce bias and improve the consistency of results. Thirdly, this study was a retrospective study, which may have involved selection bias.
Conclusions
Our study demonstrated that venous collateral status, in addition to arterial collateral status, is an important supplementary radiological index for predicting clinical outcomes of AIS. The 4D-VCS may be useful to evaluate the prognosis of patients with AIS after EVTs. Further large-scale and prospective studies are needed to confirm these results.
Acknowledgments
Funding: This work was supported by the 2020 SKY Imaging Research Fund of the Chinese International Medical Foundation (No. Z-2014-07-2003-02), the 2020 Beijing Hospital “National Natural Science Foundation of China Preliminary Research Project” (No. BJ-2020-131), and the Ningbo Medical Science and Technology Project (No. 2020Y02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-245/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-245/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Beijing Hospital approved the study, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sinha A, Stanwell P, Killingsworth MC, Bhaskar SMM. Prognostic accuracy and impact of cerebral collateral status on clinical and safety outcomes in acute ischemic stroke patients receiving reperfusion therapy: a systematic meta-analysis. Acta Radiol 2022; Epub ahead of print. [Crossref] [PubMed]
- Ravindran AV, Killingsworth MC, Bhaskar S. Cerebral collaterals in acute ischaemia: Implications for acute ischaemic stroke patients receiving reperfusion therapy. Eur J Neurosci 2021;53:1238-61. [Crossref] [PubMed]
- Zhan YH, Chen YK, Li RX, Luo GP, Wu ZQ, Liu YL, Xiao WM, Hu WD, Xie CQ. Cortical Venous Changes on Susceptibility-Weighted Imaging Predict the Cerebral Collateral Circulation as Confirmed by Digital Subtraction Angiography. Front Neurol 2021;12:691430. [Crossref] [PubMed]
- Tong LS, Guo ZN, Ou YB, Yu YN, Zhang XC, Tang J, Zhang JH, Lou M. Cerebral venous collaterals: A new fort for fighting ischemic stroke? Prog Neurobiol 2018;163-164:172-93. [Crossref] [PubMed]
- Parthasarathy R, Kate M, Rempel JL, Liebeskind DS, Jeerakathil T, Butcher KS, Shuaib A. Prognostic evaluation based on cortical vein score difference in stroke. Stroke 2013;44:2748-54. [Crossref] [PubMed]
- Zhang S, Lai Y, Ding X, Parsons M, Zhang JH, Lou M. Absent Filling of Ipsilateral Superficial Middle Cerebral Vein Is Associated With Poor Outcome After Reperfusion Therapy. Stroke 2017;48:907-14. [Crossref] [PubMed]
- Bhaskar S, Bivard A, Parsons M, Nilsson M, Attia JR, Stanwell P, Levi C. Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: Associations with collateral status. J Cereb Blood Flow Metab 2017;37:671-82. [Crossref] [PubMed]
- Zhang S, Zhang R, Jin B, Shi Z, Li C, Yu Y, Wang Z. Absent filling of the superficial middle cerebral vein is associated with reperfusion but not parenchymal hematoma in stroke patients undergoing thrombectomy: an observational study. Ann Transl Med 2020;8:1410. [Crossref] [PubMed]
- Shimonaga K, Matsushige T, Takahashi H, Hashimoto Y, Mizoue T, Ono C, Kurisu K, Sakamoto S. Early venous filling after reperfusion therapy in acute ischemic stroke. J Stroke Cerebrovasc Dis 2020;29:104926. [Crossref] [PubMed]
- Lin J, Cheng Z, Shi Y, Cai X, Huang L. Evaluating the Velocity and Extent of Cortical Venous Filling in Patients With Severe Middle Cerebral Artery Stenosis or Occlusion. Front Neurol 2021;12:610658. [Crossref] [PubMed]
- Myint MZ, Yeo LL, Tan BYQ. The EZ, Lim MC, Sia CH, Teoh HL, Sharma VK, Chan B, Ahmad A, Paliwal P, Gopinathan A, Yang C, Makmur A, Andersson T, Arnberg F, Holmin S. Internal cerebral vein asymmetry is an independent predictor of poor functional outcome in endovascular thrombectomy. J Neurointerv Surg 2022;14:683-7. [Crossref] [PubMed]
- Parthasarathy R, Sohn SI, Jeerakathil T, Kate MP, Mishra SM, Nambiar VK, Ahmad A, Menon BK, Shuaib A. A Combined Arterial and Venous Grading Scale to Predict Outcome in Anterior Circulation Ischemic Stroke. J Neuroimaging 2015;25:969-77. [Crossref] [PubMed]
- Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis GM, Broocks G, Flottmann F, Marks MP, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Heit JJ. Favorable Venous Outflow Profiles Correlate With Favorable Tissue-Level Collaterals and Clinical Outcome. Stroke 2021;52:1761-7. [Crossref] [PubMed]
- Cao R, Qi P, Liu Y, Ma X, Shen Z, Chen J. Improving Prognostic Evaluation by 4D CTA for Endovascular Treatment in Acute Ischemic Stroke Patients: A Preliminary Study. J Stroke Cerebrovasc Dis 2019;28:1971-8. [Crossref] [PubMed]
- Tan BY, Wan-Yee K, Paliwal P, Gopinathan A, Nadarajah M, Ting E, Venketasubramanian N, Seet RC, Chan BP, Teoh HL, Rathakrishnan R, Sharma VK, Yeo LL. Good Intracranial Collaterals Trump Poor ASPECTS (Alberta Stroke Program Early CT Score) for Intravenous Thrombolysis in Anterior Circulation Acute Ischemic Stroke. Stroke 2016;47:2292-8. [Crossref] [PubMed]
- Rava RA, Snyder KV, Mokin M, Waqas M, Allman AB, Senko JL, Podgorsak AR, Shiraz Bhurwani MM, Hoi Y, Siddiqui AH, Davies JM, Levy EI, Ionita CN. Assessment of a Bayesian Vitrea CT Perfusion Analysis to Predict Final Infarct and Penumbra Volumes in Patients with Acute Ischemic Stroke: A Comparison with RAPID. AJNR Am J Neuroradiol 2020;41:206-12. [Crossref] [PubMed]
- Wei LM, Zhu YQ, Lu HT, Zhao JG. Thin-slab maximum intensity projection of CT angiography for collateral score and clot burden score evaluation: comparison with conventional CT angiography. Quant Imaging Med Surg 2022;12:1163-71. [Crossref] [PubMed]
- Dziadkowiak E, Chojdak-Łukasiewicz J, Guziński M, Noga L, Paradowski B. The Usefulness of the TOAST Classification and Prognostic Significance of Pyramidal Symptoms During the Acute Phase of Cerebellar Ischemic Stroke. Cerebellum 2016;15:159-64. [Crossref] [PubMed]
- Kremenova K, Holesta M, Peisker T, Girsa D, Weichet J, Lukavsky J, Malikova H. Is limited-coverage CT perfusion helpful in treatment decision-making in patients with acute ischemic stroke? Quant Imaging Med Surg 2020;10:1908-16. [Crossref] [PubMed]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95. [Crossref] [PubMed]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. [Crossref] [PubMed]
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18. [Crossref] [PubMed]
- Lucitti JL, Mackey JK, Morrison JC, Haigh JJ, Adams RH, Faber JE. Formation of the collateral circulation is regulated by vascular endothelial growth factor-A and a disintegrin and metalloprotease family members 10 and 17. Circ Res 2012;111:1539-50. [Crossref] [PubMed]
- Bhaskar S, Bivard A, Stanwell P, Attia JR, Parsons M, Nilsson M, Levi C. Association of Cortical Vein Filling with Clot Location and Clinical Outcomes in Acute Ischaemic Stroke Patients. Sci Rep 2016;6:38525. [Crossref] [PubMed]
- Kortman HG, Smit EJ, Oei MT, Manniesing R, Prokop M, Meijer FJ. 4D-CTA in neurovascular disease: a review. AJNR Am J Neuroradiol 2015;36:1026-33. [Crossref] [PubMed]



