
A rare presentation of primary lateral ventricle H3 K27-altered diffuse midline glioma in a 14-year-old girl: a case description
Introduction
Diffuse midline glioma (DMG), H3 K27M-mutant is a type of diffuse glioma first presented in the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System, based on its histological and molecular characteristics (1). In the 2021 updated guideline, in order to recognize the alternative mechanisms by which the pathogenic pathway can be altered in these tumors, DMG is now designated as “H3 K27-altered” rather than “H3 K27M-mutant” (2). DMG, H3 K27-altered, is a WHO grade IV tumor with a poor prognosis and a 5-year overall survival rate of less than 1% (3,4). DMG commonly occurs in the brainstem, thalamus, and spinal cord (5). DMG, H3 K27-altered, rarely occurs in the lateral ventricles, with two adult cases reported in previous studies (5,6). Our patient has an extremely rare type of pediatric primary DMG, H3 K27-altered, that occurred in the lateral ventricles. Owing to its intraventricular location, the tumor was thought to be a central neurocytoma. Based on the imaging findings, surgical resection was performed by the treatment team, but the pathological characteristics were different. Hence, this case should be reported to shed new light on children with primary DMG, H3 K27-altered, in the lateral ventricles.
Case presentation
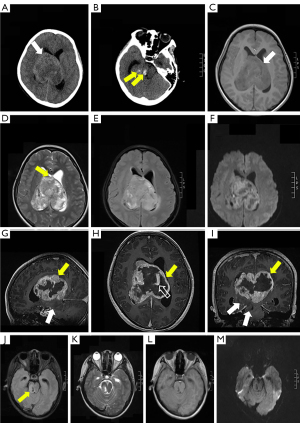
A 14-year-old girl was admitted to our hospital due to complaints of drowsiness and strabismus. Neurological examination results were normal. Axial non-enhanced computed tomography (CT) scan showed a round mass in the lateral ventricles (Figure 1A) measuring 7.7×7.5×6.6 cm3, with blurred boundaries and multiple low-density foci. Patchy areas of hyperdensity consistent with intratumoral hemorrhage were noted (Figure 1B). The lateral and third ventricles were dilated. An irregular mass was detected on gadolinium-enhanced brain magnetic resonance imaging (MRI) scan. Heterogeneous enhancement was noted in the lateral ventricle with T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) that showed hypo/isointense signals, hyper/hypointense signals, hyperintense signals, and restricted signals, respectively (Figure 1C-1I). However, the mass involved a small non-enhanced part of the right thalamus and brainstem with low signal intensity on T1WI, high signal intensity on T2WI/FLAIR, and no significant diffusion restriction on DWI (Figure 1J-1M). The midline structure was eroded and penetrated by the tumor (Figure 1D; black arrow). The lesions were characterized by dilated vessels. In the supplemental video, the largest layer of the segmented tumor can be observed, and the brain stem lesion was part of the tumor that primarily developed in the lateral ventricles (Video 1).
As it was a large symptomatic tumor, surgical resection was performed using the right frontal transcortical approach under MRI guidance. Prior to surgery, cerebrovascular digital subtraction angiography (DSA) was performed to determine the relationship between the arterial blood supply, venous sinuses, and tumor. Results of the cerebrovascular DSA revealed that multiple branches of the right anterior choroidal artery and right posterior choroidal artery supplied the lateral ventricle tumor, and the tumor had extremely rich blood supply. Given the small-caliber tumor vasculature, embolization was not performed. Owing to the hemorrhagic nature of the tumor and tight adherence to the ventricular wall, subtotal resection was carried out. Postoperatively, the patient experienced left-side hemiparesis. Unfortunately, as the lesion was highly malignant, the patient died a month after surgery. The patient only survived for more than a month from the time that the lesion was discovered.
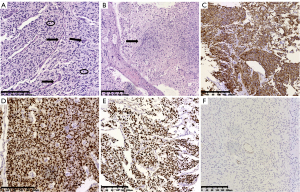
Results of the histopathological examination with hematoxylin-eosin (HE) staining showed heterotypic astrocyte-like tumor cells with elliptical or short spindle-shaped nuclei, mitotic activity, microvascular proliferation, and necrosis. The tumor cells diffusely infiltrated the adjacent brain structures. Immunohistochemistry with EnVision staining showed that the tumor cells expressed S100, glial fibrillary acidic protein (GFAP), oligodendrocyte transcription factor 2 (OLIG2), and H3 K27M but not H3 K27Me3 (Figure 2A-2F). The altered histone H3 gene was H3F3A, and the final diagnosis was DMG, H3 K27-altered (WHO grade IV).
Next-generation sequencing was performed on the foci of the lateral ventricles. Results showed that the patient harbored an H3-3A missense mutation, p.K28M; fibroblast growth factor receptor 4 (FGFR4) missense mutation, p.S632L; zinc finger-containing transcription factors 2 (GLI2) missense mutation, p.R1382C; NAD(P)H: quinone oxidoreductase 1 (NQO1) nonsense mutation, p.R201*; cyclin dependent kinase 4 (CDK4) copy number amplification, 12q14.1; and BCL2 like 11 (BCL2L11) loss of heterozygosity, c.394+1479_394+4381del (Table 1). The tumor mutation burden, which represents the number of single nucleotide protein-altering mutations per million base pairs, was 5.3 mutations per million base pairs.
Table 1
| Gene | Mutation | Mutant type |
|---|---|---|
| CDK4 | Copy number amplification | – |
| H3-3A | p.K28M missense mutation in exon 2 | c.83A>T (p.K28M) |
| FGFR4 | p.S632L missense mutation in exon 16 | c.1895C>T (p.S632L) |
| GLI2 | p.R1382C missense mutation in exon 14 | c.4144C>T (p.R1382C) |
| NQO1 | p.R201* nonsense mutation in exon 6 | c.601C>T (p.R201*) |
| BCL2L11 | Heterozygous deletion polymorphism | c.394+1479_394+4381del |
*, code termination. CDK4, cyclin dependent kinase 4; H3-3A, H3-3A gene; FGFR4, fibroblast growth factor receptor 4; GLI2, zinc finger-containing transcription factors 2; NQO1, NAD(P)H: quinone oxidoreductase 1; BCL2L11, BCL2 like 11.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We present an extremely rare case of primary lateral ventricle DMG, H3 K27-altered. A pediatric-type DMG, H3 K27-altered, mainly includes diffuse intrinsic pontine glioma (DIPG), thalamic glioma, and spinal glioma. It is a high-grade malignant tumor of the central nervous system with median survival of 10 months (7). The molecular feature of DMG is K27M mutation in the histone H3 gene H3F3A or HIST1H3B (1). In the present case, the K27M mutation occurred in the H3F3A gene, which encodes histone H3 mutant H3.3. A previous study suggested that H3.3-K27M could destroy the bivalent chromosome domain and promote tumor formation in the neural stem cells (8).
On MRI examination, the tumors are mainly located in the thalamus, brainstem, and spinal cord, and tumor space-occupying effects can be observed (5). The tumors typically appear iso- or hypointense on T1WI and hyperintense on T2WI. The signal intensity is often relatively homogeneous on FLAIR images. Intratumoral hemorrhages may occur, and necrosis is common and often shows a ring-enhanced pattern. DWI often shows diffusion-restricted and invasive growths. Other typical MRI features include involvement of the ventral side of the pons, surrounding the basilar artery. The enhancement patterns vary, but lesion enhancement is frequently not significant (9-11). The typical histology of DIPG is fibrous astrocytoma, in which cell anaplasia, increased mitotic activity, necrosis, and vascular proliferation are common (12). The imaging and histological features of this case were mostly consistent with the typical manifestations of DIPG. The difference between this case and a typical tumor was the tumor location. In addition, large vascular and multiple hemorrhages were observed in the tumor, which may be due to the direct contact of the tumor to the choroid plexus vessels in the lateral ventricles during growth, allowing arteries to supply sufficient blood to the tumor. Interestingly, the lesion showed two kinds of enhanced patterns: an obviously enhanced part in the lateral ventricles and a non-enhanced part in the right thalamus and brainstem, which may be due to intratumoral hemorrhage and necrosis.
In this case, the video of the segmented tumor clearly showed that the biggest layer of the tumor was still in the lateral ventricles. Therefore, the brain stem lesion was part of the tumor that primarily developed in the lateral ventricles. The tumor was mainly located in the lateral ventricles and involved a small part of the right thalamus and brainstem. Therefore, we believe that the lesion may originate from the structures of the lateral ventricles, such as the septum pellucidum and lateral ventricle wall. Tumors in the lateral ventricle are usually benign or low-grade malignant tumors, mainly central neurocytoma, subependymoma, and low-grade malignant glioma (13). Due to its intraventricular location, the tumor was thought to be a central neurocytoma prior to surgery. Central neurocytoma originates from the septum pellucidum or ventricular wall. On an imaging scan, it is usually detected in the lateral ventricle and connected to the septum pellucidum with a wide base, which has multiple necroses and abundant blood supply (14,15). However, imaging showed multiple cystic lesions in the central neurocytoma, which were not observed in our case (14,15). We did not observe that the lesion in the brainstem was part of the ventricular mass until the results of histopathological analysis were obtained.
High-grade DMG rarely occurs in the lateral ventricle. To date, two cases of primary lateral ventricle DMG, H3 K27-altered, have been reported (Table 2) (5,6). The first reported study included 120 cases of DMG, of which only one was a primary lateral ventricle DMG, H3 K27-altered (5). The mean age ± standard deviation (SD) was 40.63±21.82 years. In this study, patients with DMG, H3 K27-altered, had a tumor in an unusual location (including one in the lateral ventricle, two in the cerebellum, three in the corpus callosum, one in the frontal lobe, and one in the temporal lobe) and had a better prognosis than those with tumors in the brainstem (P=0.03). The second study was a case report (6). The patient was a 38-year-old adult man who experienced numbness in the right limbs and face for 2 years. The contrast-enhanced head MRI scan showed irregular mass in the left thalamus and left lateral ventricle, which did not cross the septum pellucidum. The left lateral ventricle enlarged, while the midline shifted to the right; hence, surgical resection was performed. Pathological results confirmed the diagnosis of DMG, H3 K27-altered. Based on these three cases, bilateral ventricles DMG, H3 K27-altered, could develop not only in children but also in adults. Its clinical symptoms and signs related to the tumor site progressed rapidly, and the disease course in pediatric patients was relatively short. Results of the initial MRI examination indicated that the mass was large and characterized by diffuse infiltrative growth and appeared hyperintense on FLAIR images. The lesion within the lateral ventricles demonstrated irregular peripheral enhancement easily involving the brainstem in children and thalamus in adults, which could manifest as non-enhanced and enhanced mass, respectively. If these characteristics were coincident, there was a strong likelihood that the patient had a DMG, H3 K27-altered.
Table 2
| No. | Authors & year | Age (years) | Sex | Study design & no. of DMG | Lateral ventricle | Gene alerted |
|---|---|---|---|---|---|---|
| 1 | Wang et al., 2018 (5) | Mean ± SD: 40.63±21.82 | ND | Retrospective study, 120 | 3 | H3 K27 [1] |
| H3 wild-type [2] | ||||||
| 2 | Luo et al., 2020 (6) | 38 | M | Case report, 1 | 1 | H3 K27 |
| 3 | Zhao et al., 2022 (present study) | 14 | F | Case report, 1 | 1 | H3 K27 |
[1] and [2] are the numbers of patients with H3 K27 and H3 wild-type, respectively. DMG, diffuse midline glioma; SD, standard deviation; ND, no data; M, male; F, female.
DMG, H3 K27-altered, occurs in both children and adults. The duration of clinical symptoms is similar between adults and children, but the long-term quality of life and survival time of adults is better than that of children (12). In addition, adult DMG commonly occurs in the thalamus, whereas pediatric DMG commonly occurs in the brainstem; the average tumor diameter is significantly larger in children than in adults (12). However, tumor enhancement, cystic changes, and cerebral edema were similar between adults and children (10,11).
Due to the special location of the tumor, patients with DMG, H3 K27-altered, are not suitable to undergo surgical resection or biopsy. Imaging examination, especially MRI, plays an important role in diagnosing DMG, H3 K27-altered, determining the lesion area, making a differential diagnosis, guiding biopsy, and evaluating the treatment response. Some studies have reported that imaging is related to the survival of patients with DMG, H3 K27-altered. For example, extrapontine infiltration, increased volume, enhancement, necrosis, limitation of diffusion, and distant metastasis of tumors on MRI scan are related to the patient’s survival (9). Magnetic resonance spectroscopy (MRS) was used to determine the ratio of choline to N-acetylaspartate, which can also predict the patient’s survival. A higher ratio indicates shorter patient survival, and an increase in cerebral blood volume is associated with worse prognosis (16). In addition, a high apparent diffusion coefficient skewness is closely related to a short progression-free survival (17). Tumor perfusion assessed by MRI can also provide valuable information about the tumor microvascular status and treatment response. During treatment, an increase in tumor perfusion and a decrease in tumor volume indicate a longer progression-free survival (18).
This case report has some limitations. First, the patient did not undergo MRS. Second, the patient was lost to follow-up as she died a month after surgery. Third, a connection was not found between imaging features and DMG, H3 K27-altered histone changes, owing to the lack of relevant research.
In summary, we report an extremely rare case of primary lateral ventricle DMG, H3 K27-altered, that developed in a 14-year-old girl. Owing to its intraventricular location, a presumed diagnosis of central neurocytoma was made based on the imaging characteristics, which proved to be incorrect. The patient experienced drowsiness and strabismus. Axial CT, MRI, and three-dimensional (3D) reconstruction images showed that the tumor was located in the lateral ventricles, involving a small part of the right thalamus and brainstem. 3D segmentation technology is very helpful for identifying the location of brain tumors. Surgical resection was performed as the patient had a symptomatic large tumor. Radiochemotherapy would have been provided as initial treatment if the accurate diagnosis was obtained prior to surgery. We also evaluated and summarized two other reported cases of adult primary lateral ventricle DMG, H3 K27-altered. In the future, advanced radiogenomics, and artificial intelligence could be used for further investigations. As a rare case of primary lateral ventricle DMG, H3 K27-altered, occurring in a pediatric patient, it provides new ideas for diagnosing primary DMG in the lateral ventricles of children as well as new clinical, imaging, and pathological data for further study.
Acknowledgments
The authors are grateful to the patient and his family for their kind cooperation. Additionally, we would like to thank Editage (www.editage.cn) for English language editing.
Funding: This study was supported by funding from National Natural Science Foundation of China (General Program No. 81871405); National Key Research and Development Program of China (No. 2018YFC0116404); Hospital Military Medical Research (No. 2019CZJS106).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-339/coif). All authors report that this study was supported by funding from National Natural Science Foundation of China (General Program No. 81871405); National Key Research and Development Program of China (No. 2018YFC0116404); Hospital Military Medical Research (No. 2019CZJS106). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231-51. [Crossref] [PubMed]
- Vitanza NA, Biery MC, Myers C, Ferguson E, Zheng Y, Girard EJ, et al. Optimal therapeutic targeting by HDAC inhibition in biopsy-derived treatment-naïve diffuse midline glioma models. Neuro Oncol 2021;23:376-86. [Crossref] [PubMed]
- Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022;603:934-41. [Crossref] [PubMed]
- Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, Wei Y, Hu Z, Zhao L, Teng L, Lu D. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol 2018;78:89-96. [Crossref] [PubMed]
- Luo Y, Zeng L, Xie XQ, Wang F, Liu YZ, Kang JB, Li XF, Wu DB, Qu BL. H3K27M mutant diffuse midline glioma: a case report. Eur Rev Med Pharmacol Sci 2020;24:2579-84. [PubMed]
- Bouche G, Bouffet E, Vandeborne L, Capistrano R, André N. Diffuse intrinsic pontine glioma: a clinic in Mexico, social media, and unpublishable data. Lancet Oncol 2021;22:595-6. [Crossref] [PubMed]
- Haag D, Mack N, Benites Goncalves da Silva P, Statz B, Clark J, Tanabe K, Sharma T, Jäger N, Jones DTW, Kawauchi D, Wernig M, Pfister SM. H3.3-K27M drives neural stem cell-specific gliomagenesis in a human iPSC-derived model. Cancer Cell 2021;39:407-22.e13. [Crossref] [PubMed]
- Leach JL, Roebker J, Schafer A, Baugh J, Chaney B, Fuller C, et al. MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the International DIPG Registry. Neuro Oncol 2020;22:1647-57. [Crossref] [PubMed]
- Hohm A, Karremann M, Gielen GH, Pietsch T, Warmuth-Metz M, Vandergrift LA, Bison B, Stock A, Hoffmann M, Pham M, Kramm CM, Nowak J. Magnetic Resonance Imaging Characteristics of Molecular Subgroups in Pediatric H3 K27M Mutant Diffuse Midline Glioma. Clin Neuroradiol 2022;32:249-58. [Crossref] [PubMed]
- López-Pérez CA, Franco-Mojica X, Villanueva-Gaona R, Díaz-Alba A, Rodríguez-Florido MA, Navarro VG. Adult diffuse midline gliomas H3 K27-altered: review of a redefined entity. J Neurooncol 2022;158:369-78. [Crossref] [PubMed]
- Zheng L, Gong J, Yu T, Zou Y, Zhang M, Nie L, Chen X, Yue Q, Liu Y, Mao Q, Zhou Q, Chen N. Diffuse Midline Gliomas With Histone H3 K27M Mutation in Adults and Children: A Retrospective Series of 164 Cases. Am J Surg Pathol 2022;46:863-71. [Crossref] [PubMed]
- Solomon DA, Korshunov A, Sill M, Jones DTW, Kool M, Pfister SM, et al. Myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle is defined by a recurrent PDGFRA p.K385 mutation and DNT-like methylation profile. Acta Neuropathol 2018;136:339-43. [Crossref] [PubMed]
- Steinsiepe VK, Frick H, Jochum W, Fournier JY. Differential Diagnosis of Central Neurocytoma: Two Cases. J Neurol Surg A Cent Eur Neurosurg 2021;82:599-603. [Crossref] [PubMed]
- Shah A, Vutha R, Hawaldar A, Goel A A. 22-Year Course of a Case with Central Neurocytoma. Neurol India 2022;70:1314-5. [PubMed]
- Hipp SJ, Steffen-Smith E, Hammoud D, Shih JH, Bent R, Warren KE. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol 2011;13:904-9. [Crossref] [PubMed]
- Zukotynski KA, Vajapeyam S, Fahey FH, Kocak M, Brown D, Ricci KI, Onar-Thomas A, Fouladi M, Poussaint TY. Correlation of 18F-FDG PET and MRI Apparent Diffusion Coefficient Histogram Metrics with Survival in Diffuse Intrinsic Pontine Glioma: A Report from the Pediatric Brain Tumor Consortium. J Nucl Med 2017;58:1264-9. [Crossref] [PubMed]
- Sedlacik J, Winchell A, Kocak M, Loeffler RB, Broniscer A, Hillenbrand CM. MR imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 2013;34:1450-5. [Crossref] [PubMed]


