
Coexistence of tuberculosis and sarcoidosis: a description of two cases
Introduction
Tuberculosis is an infectious diseases that presents with the pathological characteristics of granuloma and caseous necrosis caused by Mycobacterium tuberculosis (MTB) infection (1). Sarcoidosis is a noncaseous necrotizing epithelial granulomatous inflammatory disease that mainly invades the lung parenchyma and affects multiple organs throughout the body, such as the lymph nodes and skin (2). Tuberculosis and sarcoidosis rarely occur simultaneously, but there are significant similarities in clinical symptoms, imaging, and pathology between the two, making it difficult to perform differential diagnosis and thereby increasing the rate of misdiagnosis (3). In countries with a high burden of tuberculosis, the concurrent occurrence of sarcoidosis and tuberculosis needs to be carefully monitored.
Case description
Clinical data
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
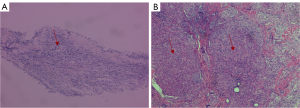
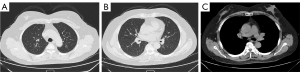
Case 1: a 43-year-old female patient underwent a chest computed tomography (CT) scan at a local hospital after 3 months of coughing and expectorating. The scan revealed multiple patchy bands and nodules in both lungs, as well as enlarged mediastinal lymph nodes. The local hospital considered the condition to be a common lung infection and administered anti-infection treatment. As the symptoms did not worsen significantly, no further diagnosis or treatment was provided. Her symptoms of cough and expectoration worsened. A chest CT scan (Figure 1) on October 20, 2023, showed multiple nodular shadows mainly along the bronchi in the upper lobes of both lungs, multiple lymph nodes in the mediastinum, and multiple slightly low-density shadows in the liver and spleen. Subsequently, the patient was admitted to The Second Hospital of Nanjing with suspected pulmonary tuberculosis. The patient is a teacher and at the time, had no chronic diseases and no history of contact with epidemic areas, industrial poisons, dust, or radioactive substances. Her serum angiotensin-converting enzyme (SACE) level was 41.1 U/L (normal range, 5–52 U/L), her tuberculosis infection T-cell test (T-spot) was negative, and her purified protein derivative (PPD) test was positive. Electronic bronchoscopy examination revealed congestion of the right bronchial mucosa in water, with multiple small nodules visible in the lumen mucosa and small nodules visible in the lumen mucosa of the left lower lobe of the lung. A biopsy was performed in the right main bronchus, and pathology (Figure 2) revealed nonnecrotic epithelioid granulomatous inflammation, with negative results from acid-fast staining and negative periodic acid-Schiff (PAS) staining. The smear and culture test of tuberculosis in the lavage fluid was negative, while MTB and resistance to rifampin in the lavage fluid was positive, and rifampicin-resistant MTB was not detected. Positron emission tomography-CT was suggested to assess the involvement of other organs, but the patient refused. Therefore, a diagnosis of secondary pulmonary tuberculosis combined with stage II pulmonary sarcoidosis was made. and the patient was administered 0.3 g of isoniazid (once a day), 0.45 g of rifampicin (once a day), 0.75 g of ethambutol (once a day), 0.5 g of pyrazinamide (3 times a day) orally, in addition to 30 mg of prednisone (once a day). After 2 months of treatment, chest CT (Figure 3) showed significant absorption of the lesion. An electronic bronchoscopy examination revealed complete subsidence of multiple small nodules in each lumen, and there was a significant improvement in the mucosal hyperemia and edema of the bronchial mucosa. Isoniazid (0.3 g, once a day) and rifampicin (0.45 g, once a day) were administered to the maintain antituberculosis treatment. The dosage of prednisone was gradually reduced and discontinued 6 months later. As of this writing, no recurrence has been observed during follow-up of lavage fluid culture of MTB or chest CT.

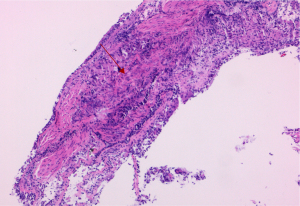

Case 2: a 50-year-old female experienced dysphagia (difficulty swallowing) for over a month. The dysphagia had begun without obvious cause and had gradually worsened. Initially, the patient could eat a small amount of food, but later, she could only take a liquid diet and was experiencing right upper abdominal pain, which worsened after eating. She was admitted to local hospital, no abnormalities were found in routine blood, urine, or fecal testing, while C-reactive protein, coagulation function, biochemical examination, blood transfusion, and antinuclear antibodies were also normal. Her clinical tests indicated the following: erythrocyte sedimentation rate 30 mm/h (reference value 0–20 mm/h), T-spot positive, and PPD test negative. Ultrasound examination showed enlargement of the bilateral axillary lymph nodes, bilateral supraclavicular lymph nodes, and bilateral inguinal lymph nodes. Puncture biopsy of the left inguinal lymph node revealed a nonnecrotic epithelioid granulomatous nodule with a multinucleated giant cell reaction. The possibility of sarcoidosis and tuberculosis infection should to be excluded based on clinical characteristics. Consequently, the patient was admitted to The Second Hospital of Nanjing for further diagnosis and treatment. The patient, a farmer by occupation, had no chronic diseases and no history of contact with epidemic areas, industrial poisons, dust, or radioactive substances. After admission, the SACE level was 180.7 U/L (reference value 5–52 U/L). Gastroscopy indicated chronic gastritis, and a mucosal biopsy of the gastric antrum showed mild chronic superficial inflammation, acid-fast staining negative, PAS negative, and Cytomegalovirus (CMV) negative. The contrast-enhanced CT scan of the neck, chest, and abdomen (Figure 4) revealed multiple nodules in both lungs. Tuberculosis needed to be excluded via analysis of the patient’s clinical examination results. Enlarged lymph nodes were observed in the bilateral clavicle area, mediastinum, bilateral hilar and hepatic regions, and bilateral inguinal areas. Small lymph nodes were also detected around the cervical vascular sheath and adjacent to the main abdominal artery, with multiple low-density lesions in the spleen, suggesting the potential for the extrapulmonary involvement of sarcoidosis. Electronic bronchoscopy examination further revealed mucosal congestion and hypertrophy, in addition to unevenness in the trachea, bronchus, and lobar bronchus, with small nodular protrusions. Lavage was performed in the anterior segment of the right upper lobe, and further smear and culture testing on the lavage fluid revealed negative results for tuberculosis; Gene Xpert on lavage fluid yielded positive results, but testing was negative for rifampicin resistance. Biopsy of the middle lobe of the right lung and resection of a nodule in the left upper limb forearm subcutaneous were performed. The pathology results (Figure 5) showed nonnecrotic epithelioid granulomatous inflammation with multinucleated giant cells, negative acid-fast staining, and negative PAS staining in both specimens. A diagnosis of secondary pulmonary tuberculosis combined with stage II pulmonary sarcoidosis and skin sarcoidosis was made. Thus, the patient was treated with 0.3 g of isoniazid (once a day), 0.6 g of rifampicin (once a day), 0.75 g of ethambutol (once a day), and 0.5 g of pyrazinamide (three times a day) orally, along with 30 mg of prednisone (once a day), and the dosage was gradually reduced. After treatment, a chest CT scan (Figure 6) showed significant absorption of the lesion in the upper lobe of the right lung, with significant reduction in the hilar and mediastinal lymph nodes. Electronic bronchoscopy examination revealed no hypertrophy, hyperemia, or edema in the mucous membrane of either lumen, and all small nodules had subsided. As of this writing, there has been no recurrence.

Discussion
China is among those countries with a high burden of tuberculosis. The Global Tuberculosis Report 2023 issued by the World Health Organization (WHO) indicated that there were 10.6 million new cases of tuberculosis in the world in 2022, and 780,000 new cases of tuberculosis in China, placing it the third among 30 countries with the highest burden of tuberculosis (4). The incidence of sarcoidosis is highest among African Americans and Nordic people (5), while for China, there is no detailed epidemiological data on sarcoidosis. The incidence ratio of sarcoidosis between men and women in China is 1:1.6, with the average age of those with sarcoidosis being 47.6 years (6). Among the two patients reported in this study, both were female patients with an average age of 46.5 years. The etiology of sarcoidosis is not well understood but an association with the pathogenic infection of bacteria such as tuberculosis, nontuberculosis mycobacterium, and Propionibacterium acnes has been postulated (7). One study reported that the risk of sarcoidosis in patients with tuberculosis to be 8.08 times higher than that in those without tuberculosis (8). The relationship between tuberculosis and sarcoidosis is complex. Gupta et al. conducted a meta-analysis on 31 studies that used polymerase chain reaction (PCR) analysis to assess the presence of mycobacteria in patients with sarcoidosis from 1980 to 2006, totaling 874 samples; the results revealed that 26.4% of the sarcoidosis samples tested positive for mycobacterium, indicating that MTB infection could be a shared pathophysiological link between tuberculosis and sarcoidosis (9). By analyzing the cellular and humoral immune responses of patients with tuberculosis and sarcoidosis to MTB antigen, it was found that pro-inflammatory factors, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), were increased in both patients, and other antibodies targeting tuberculosis-specific RD1 and Ag85 antigens have been detected in patients with sarcoidosis, with total reactivity ranging from 44.4% to 60%, which suggests that the immune response induced by sarcoidosis may directly target MTB infection (10).
The clinical manifestations of sarcoidosis are highly heterogeneous, varying from asymptomatic cases to progressive exacerbations and relapses with associated symptoms. The specificities in chest CT findings include bilateral hilar symmetric lymph node enlargement and/or the presence of multiple small perilymphatic nodules in the middle and upper regions of both lungs (11,12). The diagnosis of sarcoidosis is based primarily on clinical features, radiological findings, and histological evidence of nonnecrotizing granulomatous inflammation in one or more tissues, but there is a need to rule out the cause of other granulomatous diseases (13). The etiology of granulomatous diseases can be categorized into infectious and noninfectious causes. MTB stands out as the primary infectious cause, particularly in regions with high tuberculosis prevalence. Infections from fungi, parasites, and other pathogens are uncommon. Sarcoidosis is the most common non-infectious granulomatous diseases Other uncommon causes encompass granulomatous polyvasculitis, chronic berylliosis, hypersensitivity pneumonia, and granulomatous reactions induced by drugs or tumors (14,15). Sarcoidosis and tuberculosis are both granulomatous lesions, but their pathological features are different (16). Granulomas in sarcoidosis are primarily located along the lymphatic interstitium. These nodules have a consistent size with clear boundaries and do not merge. Fibrous tissue hyperplasia is common, with minimal infiltration of surrounding inflammatory cells, with the occasional presence of inclusion bodies. In tuberculosis, granulomas may or may not exhibit caseous necrosis. Varying in size, granulomatous nodules are usually distributed along the airway. There is also a pronounced increase in lymphocyte infiltration around the nodules, indicating infectious lesion characteristics (17). Hence, for diagnosing the pathological manifestations of granulomatous lesions, it is crucial to initially investigate potential sources of pathogenic infection. Additionally, obtaining a detailed medical history, including occupational history, drug exposure, and allergen exposure, is essential. Augmenting relevant laboratory examinations is necessary to eliminate the possibility of other granulomatous diseases.
Case 1 had respiratory symptoms mainly characterized by coughing, whereas case 2 had digestive symptoms mainly characterized by dysphagia, with no clinical specificity. However, the chest CT manifestations of the two patients were relatively similar. The lung lesions were mainly nodules distributed along the airway in the upper lobe of the right lung and were accompanied by bilateral hilar and mediastinal lymph node enlargement. Both patients underwent bronchoscopy with concomitant bronchial mucosal biopsies, and the pathology showed nonnecrotic epithelioid granuloma, which, in combination with the negative findings from acid-fast staining, indicated sarcoidosis. MTB pathogen detection was carried out on patient samples. The two cases tested positive on Gene Xpert testing of alveolar lavage fluid. In regions with a high incidence of tuberculosis or sarcoidosis, it is strongly recommended to routinely employ special staining (including, but not limited to, acid-fast staining) on pathologically reported granulomatous lesions to identify MTB. However, the positivity rate of acid-fast staining is low, ranging from 15.5% to 29% (16). This suggests that molecular biology techniques, such as real-time fluorescence quantitative PCR, should be used for detecting MTB in histological specimens. However, it cannot replace mycobacterium culture as the diagnostic gold standard, and positive results should be comprehensively considered in conjunction with clinical manifestations. The coexistence of tuberculosis and sarcoidosis was diagnosed based on clinical characteristics, chest imaging, etiology, and tissue biopsy of nonnecrotizing granuloma, and the absence of evidence for other causes of granuloma.
Sarcoidosis exhibits some degree of self-healing, but the occurrence of respiratory or systemic symptoms may worsen the lung lesions. Glucocorticoids can be used as the main drug for treating sarcoidosis (18). For patients with the dual characteristics of sarcoidosis and tuberculosis, antituberculosis treatment should be initiated as soon as possible, and imaging and pathogenic examinations should be conducted in time to evaluate the efficacy of the treatment. For patients showing poor response, hormone and immunosuppressant therapy should be initiated for those that meet the relevant criteria. Researchers have classified pulmonary sarcoidosis into four stages based on chest imaging findings (19), with the spontaneous remission rate of stage II pulmonary sarcoidosis accounting for 40% to 70% of all cases (20) . However, in our clinical practice, we have found that regardless of whether stage I or stage II sarcoidosis goes untreated, after several months of clinical follow-up observation, the majority of patients show no significant improvement, and only a few patients show improvement on imaging. Case 1 had clear symptoms of cough and phlegm, while case 2 exhibited evident dysphagia and a progressive worsening of symptoms. Imaging results revealed the presence of multiple enlarged lymph nodes, aligning with the criteria for hormone therapy in cases of pulmonary sarcoidosis. Therefore, in addition to antituberculosis treatment, oral prednisone was administered, and clinical symptoms appeared to significantly improve during the follow-up. Chest CT showed significant reduction in mediastinal and hilar lymph nodes, and significant absorption of lung lesions. Reexamination under tracheoscopy confirmed the disappearance of submucosal nodules in the trachea, indicating that the treatment was effective.
In summary, this report presents two rare cases of tuberculosis and pulmonary sarcoidosis coexisting, with good results following the combination of antituberculosis and glucocorticoid treatment. It is hoping our results can serve as a reference for the future management of patients with tuberculosis combined with sarcoidosis.
Acknowledgments
Funding: This study was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1696/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mise K, Goic-Barisic I, Puizina-Ivic N, Barisic I, Tonkic M, Peric I. A rare case of pulmonary tuberculosis with simultaneous pulmonary and skin sarcoidosis: a case report. Cases J 2010;3:24. [Crossref] [PubMed]
- Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet 2014;383:1155-67. [Crossref] [PubMed]
- Cho HS, Kim SJ, Yoo JY. Sarcoidosis during treatment of pulmonary tuberculosis: a rare case report and review of the literature. J Int Med Res 2021;49:3000605211001632. [Crossref] [PubMed]
- Adigun R, Singh R. Tuberculosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2024.
- Wang SH, Chung CH, Huang TW, Tsai WC, Peng CK, Huang KL, Perng WC, Chian CF, Chien WC, Shen CH. Bidirectional association between tuberculosis and sarcoidosis. Respirology 2019;24:467-74. [Crossref] [PubMed]
- Zhang J, Li Y, Xu Q, Wang H. Research progress in the diagnosis of sarcoidosis. International Journal of Respiration 2018;38:1353-7.
- Bennett D, Bargagli E, Refini RM, Rottoli P. New concepts in the pathogenesis of sarcoidosis. Expert Rev Respir Med 2019;13:981-91. [Crossref] [PubMed]
- Moravvej H, Vesal P, Abolhasani E, Nahidi S, Mahboudi F. Comorbidity of leishmania major with cutaneous sarcoidosis. Indian J Dermatol 2014;59:316. [Crossref] [PubMed]
- Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 2007;30:508-16. [Crossref] [PubMed]
- Agrawal R, Kee AR, Ang L, Tun Hang Y, Gupta V, Kon OM, Mitchell D, Zierhut M, Pavesio C. Tuberculosis or sarcoidosis: Opposite ends of the same disease spectrum? Tuberculosis (Edinb) 2016;98:21-6. [Crossref] [PubMed]
- Verleden SE, Vanstapel A, De Sadeleer L, Dubbeldam A, Goos T, Gyselinck I, Geudens V, Kaes J, Van Raemdonck DE, Ceulemans LJ, Yserbyt J, Vos R, Vanaudenaerde B, Weynand B, Verschakelen J, Wuyts WA. Distinct Airway Involvement in Subtypes of End-Stage Fibrotic Pulmonary Sarcoidosis. Chest 2021;160:562-71. [Crossref] [PubMed]
- Drent M, Crouser ED, Grunewald J. Challenges of Sarcoidosis and Its Management. N Engl J Med 2021;385:1018-32. [Crossref] [PubMed]
- Rossides M, Darlington P, Kullberg S, Arkema EV. Sarcoidosis: Epidemiology and clinical insights. J Intern Med 2023;293:668-80. [Crossref] [PubMed]
- Chopra A, Avadhani V, Tiwari A, Riemer EC, Sica G, Judson MA. Granulomatous lung disease: clinical aspects. Expert Rev Respir Med 2020;14:1045-63. [Crossref] [PubMed]
- Llanos O, Hamzeh N. Sarcoidosis. Med Clin North Am 2019;103:527-34. [Crossref] [PubMed]
- Nazarullah A, Nilson R, Maselli DJ, Jagirdar J. Incidence and aetiologies of pulmonary granulomatous inflammation: a decade of experience. Respirology 2015;20:115-21. [Crossref] [PubMed]
- Tuna T, Ozkaya S, Dirican A, Erkan L. An intracerebral mass: tuberculosis or sarcoidosis? BMJ Case Rep 2013;2013:bcr2013009570. [Crossref] [PubMed]
- Bansal S, Utpat K, Desai U, Basu S, Joshi JM. Sarcoidosis Presenting with Tracheobronchial Calcification and Nodularity: An Unusual Case Presentation with Treatment Response Assessment by (18)F-FDG-PET/CT. Indian J Nucl Med 2017;32:217-20. [Crossref] [PubMed]
- Saito W, Kobayashi H, Shinkai M, Ohara I, Mimura S, Kurumagawa T, Kanou S, Motoyoshi K. Pulmonary involvement in sarcoidosis: CT findings at diagnosis and their changes at follow-up in cases without corticosteroid treatment. Nihon Kokyuki Gakkai Zasshi 2002;40:210-4.
- Interstitial Lung Disease Group, Chinese Thoracic Society, Chinese Medical Association. Interstitial Lung Disease Working Committee, Chinese Association of Chest Physicians, Chinese Medical Doctor Association. Chinese expert consensus on the diagnosis and treatment of pulmonary sarcoidosis. Zhonghua Jie He He Hu Xi Za Zhi 2019;42:685-93. [Crossref] [PubMed]


